mp:V2.0.0 Ontwerp medicatieproces 9 ENG: verschil tussen versies
| (34 tussenliggende versies door 2 gebruikers niet weergegeven) | |||
| Regel 142: | Regel 142: | ||
#Creating a new changed MA as part of the same 'medicamenteuze behandeling'. The starting date of this new MA may also be in the future. | #Creating a new changed MA as part of the same 'medicamenteuze behandeling'. The starting date of this new MA may also be in the future. | ||
Prescribing a new medicinal product always results in a new MA. An MA is always related to a single 'medicamenteuze behandeling'. For the time being, the PRK level (Prescription Code from the G-standard) of the medicinal product determines whether the MA belongs to a new or an existing 'medicamenteuze behandeling'. This may be extended to the SNK level (Substance Name Code from the G-standard) in the future, which would mean that changes in strength or between medicinal products from the same group no longer lead to a new 'medicamenteuze behandeling'. A detailed description can be found in [[#Process step: | Prescribing a new medicinal product always results in a new MA. An MA is always related to a single 'medicamenteuze behandeling'. For the time being, the PRK level (Prescription Code from the G-standard) of the medicinal product determines whether the MA belongs to a new or an existing 'medicamenteuze behandeling'. This may be extended to the SNK level (Substance Name Code from the G-standard) in the future, which would mean that changes in strength or between medicinal products from the same group no longer lead to a new 'medicamenteuze behandeling'. A detailed description can be found in [[#Process step: Making a medication agreement|paragraph 2.2.5 Process step: Making a medicatieafspraak]]. | ||
Exceptions: | Exceptions: | ||
| Regel 257: | Regel 257: | ||
===Trigger event=== | ===Trigger event=== | ||
*Outpatient setting: consultation of and/or prescription to outpatients and patients residing in another healthcare institution<ref>This is about patients from a nursing home or a mental health institution who are going to the outpatient clinic of a hospital.</ref>. In this case, medication verification often occurs during treatment assessment (see [[#Process step: Evaluating a | *Outpatient setting: consultation of and/or prescription to outpatients and patients residing in another healthcare institution<ref>This is about patients from a nursing home or a mental health institution who are going to the outpatient clinic of a hospital.</ref>. In this case, medication verification often occurs during treatment assessment (see [[#Process step: Evaluating a pharmaceutical treatment|paragraph 2.2.4)]]. | ||
*Clinical setting: preparation of patient admission. | *Clinical setting: preparation of patient admission. | ||
===Process=== | ===Process=== | ||
| Regel 278: | Regel 278: | ||
===Information systems and transaction groups=== | ===Information systems and transaction groups=== | ||
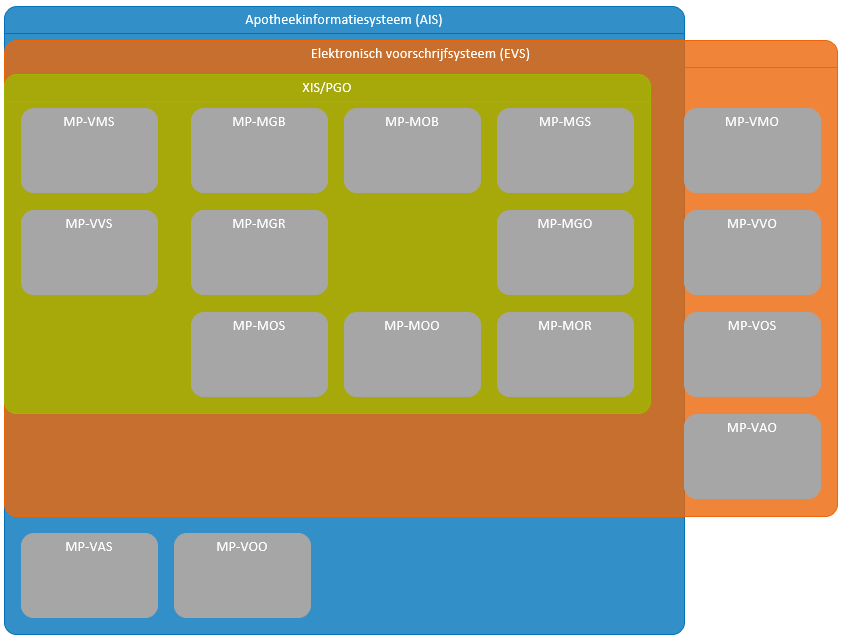
[[#Information systems and transactions|Chapter | [[#Information systems and transactions|Chapter 7]] includes an overview of all information systems, system roles, transactions, etc. Those most important for the medication verification process are included in the overview below. | ||
{{anchor|figuur 4}} | {{anchor|figuur 4}} | ||
| Regel 284: | Regel 284: | ||
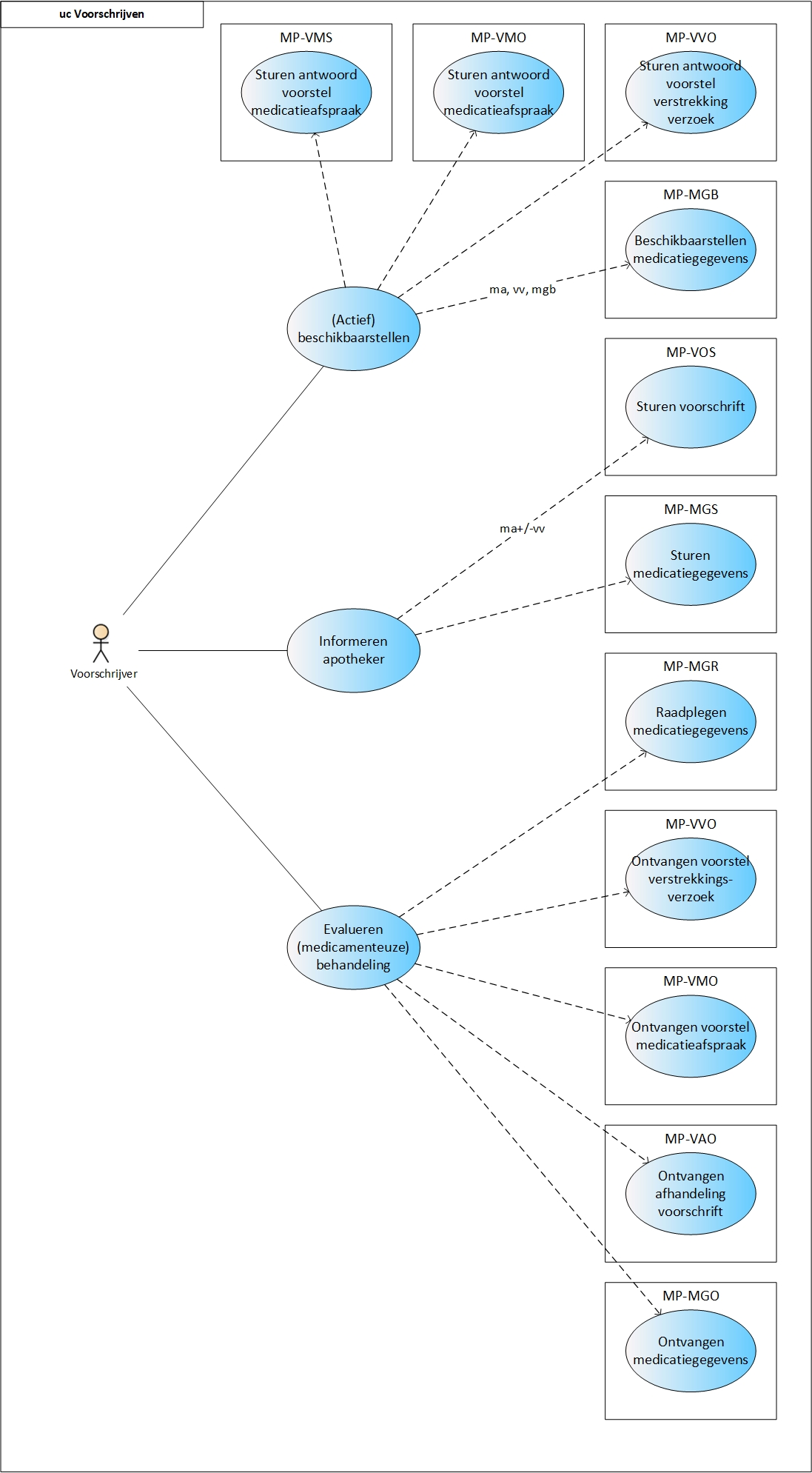
==Process: prescribe== | ==Process: prescribe== | ||
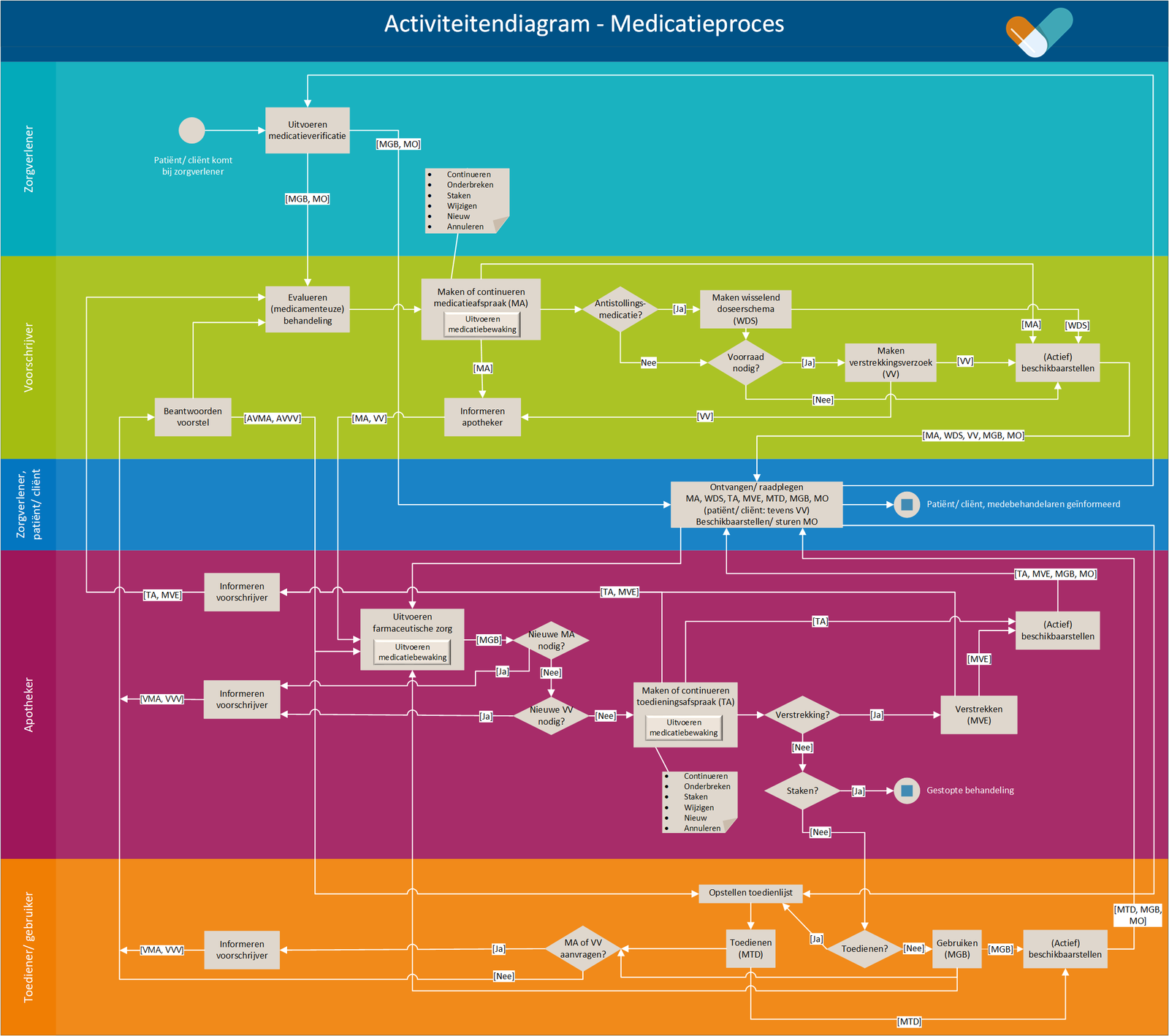
This paragraph describes the prescription process. This includes all prescribers, such as general practitioners, specialists, other physicians and specialist nurse prescribers. The prescription process consists of an evaluation of existing pharmaceutical treatment, if any. If necessary, an medication agreement is created and, only in an ambulatory situation, possibly a medication dispense request. Finally, the recorded data are (actively) made available | This paragraph describes the prescription process. This includes all prescribers, such as general practitioners, specialists, other physicians and specialist nurse prescribers. The prescription process consists of an evaluation of existing pharmaceutical treatment, if any. If necessary, an medication agreement is created and, only in an ambulatory situation, possibly a medication dispense request. Finally, the recorded data are (actively) made available. | ||
===Current situation=== | ===Current situation=== | ||
| Regel 311: | Regel 311: | ||
*approve a VMA or a VVV (including a reply via the AVVV) <br> | *approve a VMA or a VVV (including a reply via the AVVV) <br> | ||
These situations are further explained in the following paragraph. See also [[#Medicamenteuze behandeling|paragraph 1.3.3]] for more information on the concept of 'MBH'. | These situations are further explained in the following paragraph. See also [[#'Medicamenteuze behandeling'|paragraph 1.3.3]] for more information on the concept of 'MBH'. | ||
A new medication overview may be created to conclude the evaluation and the resulting new agreements and dispense requests (see [[#Inference rules|paragraph 5.6]]). | A new medication overview may be created to conclude the evaluation and the resulting new agreements and dispense requests (see [[#Inference rules|paragraph 5.6]]). | ||
| Regel 322: | Regel 322: | ||
Before the MA is made (actively) available, medication monitoring will occur in accordance with current guidelines. This is a part of this process step. | Before the MA is made (actively) available, medication monitoring will occur in accordance with current guidelines. This is a part of this process step. | ||
The next paragraphs describe the different situations in which an MA is created, i.e. initial medication agreement, continuing medication, discontinuing medication, temporarily halting medication or correcting/canceling a agreed medication. Information about 'MBH' is assumed to be known (see [[#Medicamenteuze behandeling|paragraph 1.3.3]]). | The next paragraphs describe the different situations in which an MA is created, i.e. initial medication agreement, continuing medication, discontinuing medication, temporarily halting medication or correcting/canceling a agreed medication. Information about 'MBH' is assumed to be known (see [[#'Medicamenteuze behandeling'|paragraph 1.3.3]]). | ||
====New medication agreement==== | ====New medication agreement==== | ||
A new MA is created at the start or modification of an MBH. When a new MBH is started, the prescriber should consider whether an existing MBH should be discontinued. The description in [[#Medicamenteuze behandeling|paragraph 1.3.3]] is based on the most common process from prescription to administering or using. In a transitional situation or in the absence of digital data, it is also technically possible that an MBH could start with a TA, for example. This might occur for instance when a pharmacist has not received the MA with the corresponding MBH in digital form. The pharmacist will consequently start a new MBH when the TA is created. This may also be the case for any other building block. A patient can, for example, start an MBH by recording MGB, without having the original MBH. | A new MA is created at the start or modification of an MBH. When a new MBH is started, the prescriber should consider whether an existing MBH should be discontinued. The description in [[#'Medicamenteuze behandeling'|paragraph 1.3.3]] is based on the most common process from prescription to administering or using. In a transitional situation or in the absence of digital data, it is also technically possible that an MBH could start with a TA, for example. This might occur for instance when a pharmacist has not received the MA with the corresponding MBH in digital form. The pharmacist will consequently start a new MBH when the TA is created. This may also be the case for any other building block. A patient can, for example, start an MBH by recording MGB, without having the original MBH. | ||
====Continuing medication==== | ====Continuing medication==== | ||
| Regel 334: | Regel 334: | ||
*At admission to an institution where the home medication continues to be used, whether or not in combination with self-medication. <br> | *At admission to an institution where the home medication continues to be used, whether or not in combination with self-medication. <br> | ||
In both of these cases, the existing MA will not be adjusted. Should there be a change in PRK, e.g. at admission or discharge, the existing MBH will be discontinued by creating a stop-MA (see [[#Discontinuing medication|paragraph 2.2.5.3]]) and a new MBH is started (see [[#New | In both of these cases, the existing MA will not be adjusted. Should there be a change in PRK, e.g. at admission or discharge, the existing MBH will be discontinued by creating a stop-MA (see [[#Discontinuing medication|paragraph 2.2.5.3]]) and a new MBH is started (see [[#New medication agreement|paragraph 2.2.5.1]]). | ||
====Discontinuing medication==== | ====Discontinuing medication==== | ||
| Regel 366: | Regel 366: | ||
:d) Duration of treatment (such as an extension of the therapy).<br> | :d) Duration of treatment (such as an extension of the therapy).<br> | ||
Switching to a completely different medicinal product is technically a switch to a different MBH (see also [[#Medicamenteuze behandeling|paragraph 1.3.3]]). In that case, the physician will discontinue the existing pharamceutical treatment (see [[#Discontinuing medication|paragraph 2.2.5.3]]) and start a new one (see [[#New | Switching to a completely different medicinal product is technically a switch to a different MBH (see also [[#'Medicamenteuze behandeling'|paragraph 1.3.3]]). In that case, the physician will discontinue the existing pharamceutical treatment (see [[#Discontinuing medication|paragraph 2.2.5.3]]) and start a new one (see [[#New medication agreement|paragraph 2.2.5.1]]). | ||
If the PRK stays the same, changes will be recorded under the same MBH. When a modification needs to be made, a technical stop-MA is created (see [[#Discontinuing medication|paragraph 2.2.5.3]]) and a new MA is made with the desired change. The appointment date of the technical stop-MA and the new MA must be always be the same. The reason for the modification is included in the new MA with a reference to the original MA, if possible. Modifications may take effect immediately or in the future. A technical stop-MA and the related new MA are made available simultaneously. In case of renewal of an MA of which the duration has already expired or the end date has already expired, then this is not seen as a change (a stop-MA on the already automatically stopped MA is unnecessary). In this case, a new MA under the same MBH can be made. | If the PRK stays the same, changes will be recorded under the same MBH. When a modification needs to be made, a technical stop-MA is created (see [[#Discontinuing medication|paragraph 2.2.5.3]]) and a new MA is made with the desired change. The appointment date of the technical stop-MA and the new MA must be always be the same. The reason for the modification is included in the new MA with a reference to the original MA, if possible. Modifications may take effect immediately or in the future. A technical stop-MA and the related new MA are made available simultaneously. In case of renewal of an MA of which the duration has already expired or the end date has already expired, then this is not seen as a change (a stop-MA on the already automatically stopped MA is unnecessary). In this case, a new MA under the same MBH can be made. | ||
| Regel 431: | Regel 431: | ||
This step involves information exchange. Information can be sent or made available with different intentions: | This step involves information exchange. Information can be sent or made available with different intentions: | ||
A. As an order for the pharmacist to dispense medication. The prescriber sends the MA to the pharmacist. In an ambulatory situation, the VV is also sent to the patient’s pharmacist. If the pharmacist is not known, this process step may also entail giving out a paper prescription and/or (reactively) making the data available (see C). When the pharmacist has filled the order, the prescriber will receive a notification (see [[#Information systems and transaction groups|paragraph 2.3. | A. As an order for the pharmacist to dispense medication. The prescriber sends the MA to the pharmacist. In an ambulatory situation, the VV is also sent to the patient’s pharmacist. If the pharmacist is not known, this process step may also entail giving out a paper prescription and/or (reactively) making the data available (see C). When the pharmacist has filled the order, the prescriber will receive a notification (see [[#Information systems and transaction groups 3|paragraph 2.3.10]]).<br> | ||
B. As an order for the pharmacist to implement a medication change (including stop-MA with stop type 'permanent' and 'temporary') that impacts or may impact current MVE by this pharmacist. A current MVE indicates an order (as in A) that has been accepted, but has not yet been completely filled. For example, when medication is still being dispensed or when a VV dictates that medication should be dispensed multiple times, but not all have taken place yet, e.g. with the GDS.<br> | B. As an order for the pharmacist to implement a medication change (including stop-MA with stop type 'permanent' and 'temporary') that impacts or may impact current MVE by this pharmacist. A current MVE indicates an order (as in A) that has been accepted, but has not yet been completely filled. For example, when medication is still being dispensed or when a VV dictates that medication should be dispensed multiple times, but not all have taken place yet, e.g. with the GDS.<br> | ||
C. Making medication data (MA, VV, MGB) and possibly the medication overview available to allow fellow health professionals and/or patients to access these later, possibly in combination with prescription without a specified recipient (see [[#Prescription without specified recipient (ambulatory)|paragraph | C. Making medication data (MA, VV, MGB) and possibly the medication overview available to allow fellow health professionals and/or patients to access these later, possibly in combination with prescription without a specified recipient (see [[#Prescription without specified recipient (ambulatory)|paragraph 9.1]]). <br> | ||
D. Actively sending (informing) medication data (one or more building blocks) to a specific health professional at the request of the patient when in the presence of this health professional or at discharge.<br> | D. Actively sending (informing) medication data (one or more building blocks) to a specific health professional at the request of the patient when in the presence of this health professional or at discharge.<br> | ||
| Regel 441: | Regel 441: | ||
In case of corrected data, the prescriber assesses who should be actively informed of this correction. This can be done, for example, by sending the new MA (option A or B above) or by means of a telephone consultation. | In case of corrected data, the prescriber assesses who should be actively informed of this correction. This can be done, for example, by sending the new MA (option A or B above) or by means of a telephone consultation. | ||
In an ambulatory setting (general practitioner/outpatient clinic) medication data are usually sent or made available directly; in a clinical setting, medication data is usually made available at discharge or interim leave from the institution. | In an ambulatory setting (general practitioner/outpatient clinic) medication data are usually sent or made available directly; in a clinical setting, medication data is usually made available at discharge or interim leave from the institution. | ||
This chapter deals with prescription with a specified recipient; [[#Prescription without specified recipient (ambulatory)|paragraph | This chapter deals with prescription with a specified recipient; [[#Prescription without specified recipient (ambulatory)|paragraph 9.1]] details prescription without a specified recipient. | ||
See also [[#Informing and (actively) making available|paragraph 1.3.5]] for a further explanation on informing (situation A, B, D) and making available (situation C). | See also [[#Informing and (actively) making available|paragraph 1.3.5]] for a further explanation on informing (situation A, B, D) and making available (situation C). | ||
| Regel 455: | Regel 455: | ||
The prescriber and the pharmacist as well as other health professionals and users all make use of an information system, respectively, an electronic prescribing system (EVS), a pharmacist information system (AIS, incl. ZAIS), a XIS and a PGO<ref> XIS is a generic term for a random (health care) information system. PHR=personal health records.</ref>. These information systems each have different system roles which enable the exchange of data between these information systems as part of the prescription process. | The prescriber and the pharmacist as well as other health professionals and users all make use of an information system, respectively, an electronic prescribing system (EVS), a pharmacist information system (AIS, incl. ZAIS), a XIS and a PGO<ref> XIS is a generic term for a random (health care) information system. PHR=personal health records.</ref>. These information systems each have different system roles which enable the exchange of data between these information systems as part of the prescription process. | ||
[[#Information systems and transactions|Chapter | [[#Information systems and transactions|Chapter 7]] includes an overview of all information systems, system roles, transactions, etc. The most important elements for the prescription process are included in the overview below. | ||
{{anchor|figuur 5}} | {{anchor|figuur 5}} | ||
| Regel 505: | Regel 505: | ||
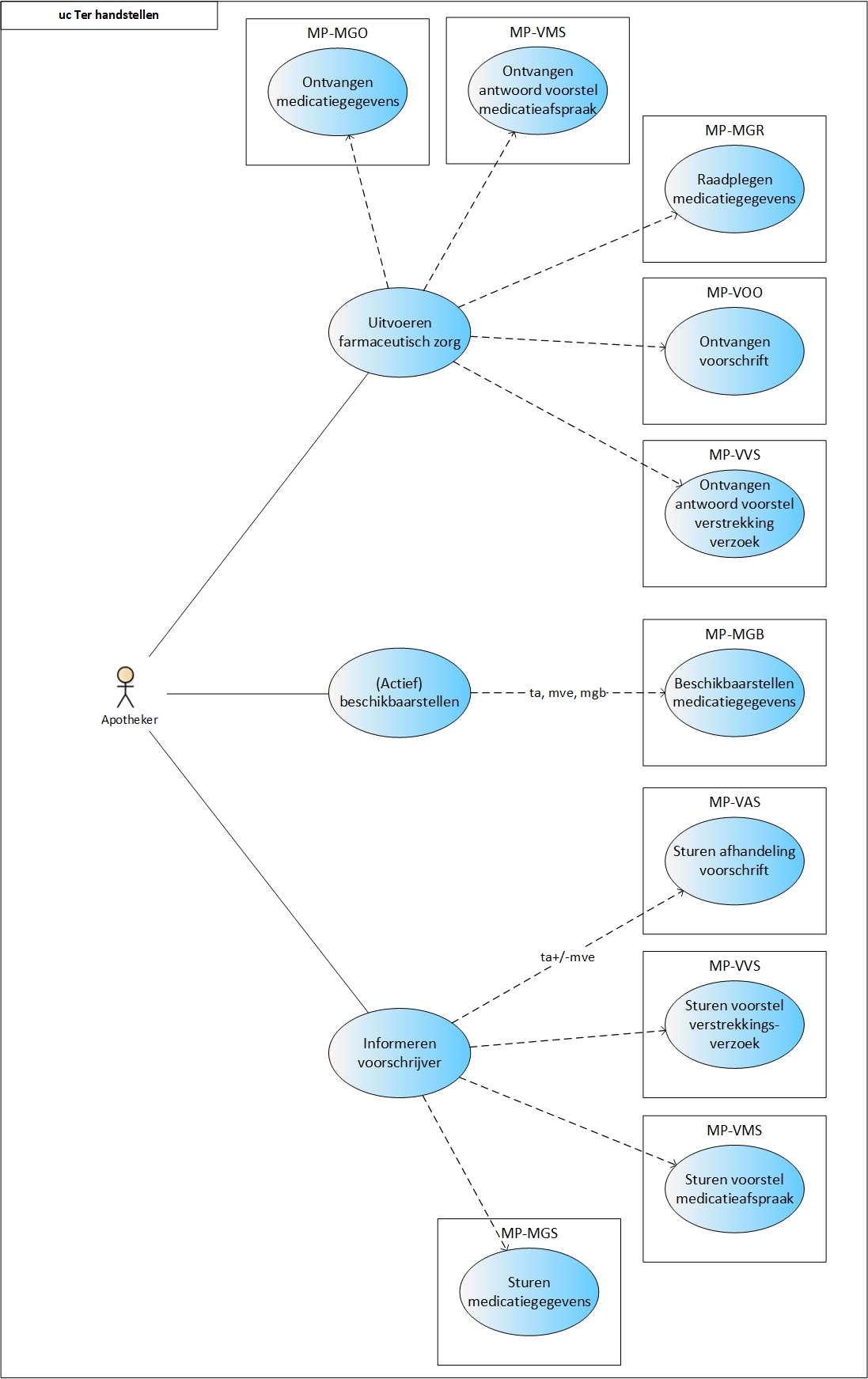
==Process: dispense== | ==Process: dispense== | ||
This paragraph describes the process of dispensing medication, including repeat prescriptions and GDS. This process encompasses all actions a pharmacist must take for the patient to not only receive a medicinal product, but to also receive the associated pharmaceutical care ensuring a safe and effective use of the medicinal product by the patient. The dispense process starts with providing pharmaceutical care. If necessary, a medication administration agreement will be made and if needed, medication is dispensed. Medication dispense (i.e. handing out a medicinal product) does not always take place. This may be the case when the medication agreement is changed (e.g., in case of dose reduction where the patient still has enough supply), when the medication is discontinued or, in an ambulatory situation, the medication is not picked up. When the MA and/or the VV do not comply (see [[#Process step: Informing the prescriber|paragraph 2.3.5]]), the prescriber will be informed. Finally, the recorded data are (actively) made available | This paragraph describes the process of dispensing medication, including repeat prescriptions and GDS. This process encompasses all actions a pharmacist must take for the patient to not only receive a medicinal product, but to also receive the associated pharmaceutical care ensuring a safe and effective use of the medicinal product by the patient. The dispense process starts with providing pharmaceutical care. If necessary, a medication administration agreement will be made and if needed, medication is dispensed. Medication dispense (i.e. handing out a medicinal product) does not always take place. This may be the case when the medication agreement is changed (e.g., in case of dose reduction where the patient still has enough supply), when the medication is discontinued or, in an ambulatory situation, the medication is not picked up. When the MA and/or the VV do not comply (see [[#Process step: Informing the prescriber|paragraph 2.3.5]]), the prescriber will be informed. Finally, the recorded data are (actively) made available. | ||
Medication assessment is the process in which the physician and pharmacist consider all medication of the patient against the background of his condition, applicable treatment guidelines, the well-being of the patient, etc. Medication assessment is defined in this document as a combination of treatment evaluation (as described in the previous paragraphs) and pharmaceutical care. Depending on the findings, the previously described medication verification and prescription processes are followed, after which medication dispense takes place. | Medication assessment is the process in which the physician and pharmacist consider all medication of the patient against the background of his condition, applicable treatment guidelines, the well-being of the patient, etc. Medication assessment is defined in this document as a combination of treatment evaluation (as described in the previous paragraphs) and pharmaceutical care. Depending on the findings, the previously described medication verification and prescription processes are followed, after which medication dispense takes place. | ||
| Regel 543: | Regel 543: | ||
*Proposing a new medication dispense (VVV). | *Proposing a new medication dispense (VVV). | ||
The last three situations are explained in the following paragraph. The first two situations are explained in [[#Process step: Creating | The last three situations are explained in the following paragraph. The first two situations are explained in [[#Process step: Creating an administration agreement|paragraph 2.3.6]]. | ||
In conclusion of provided pharmaceutical care, which may comprise new agreements and medication dispenses, a new up-to-date medication overview may be compiled and made available. | In conclusion of provided pharmaceutical care, which may comprise new agreements and medication dispenses, a new up-to-date medication overview may be compiled and made available. | ||
| Regel 559: | Regel 559: | ||
*When the temporarily halted pharmaceutical treatment may be resumed. | *When the temporarily halted pharmaceutical treatment may be resumed. | ||
*A TA is not yet created or modified in these situations. | *A TA is not yet created or modified in these situations. | ||
Instead, the pharmacist starts by contacting the prescriber by phone, informing him of the suspected error and proposing an alternative. The pharmacist may also send a digital proposition called VMA to the prescriber. He recommends a specific MA in this proposal, together with the reason and arguments for that recommendation. The prescriber may approve the VMA and make it into a final MA (see also [[#Process step: Evaluating a | Instead, the pharmacist starts by contacting the prescriber by phone, informing him of the suspected error and proposing an alternative. The pharmacist may also send a digital proposition called VMA to the prescriber. He recommends a specific MA in this proposal, together with the reason and arguments for that recommendation. The prescriber may approve the VMA and make it into a final MA (see also [[#Process step: Evaluating a pharmaceutical treatment|paragraph 2.2.4]]).<br> | ||
A new VV is needed when the patient’s medication stock is depleted or nearly used and the treatment may need to be continued (request repeat prescription). The patient either requests a repeat MVE from the pharmacist or has signed up in the past for proactive repeat MVE and a notification signal is generated by the repeat module of the AIS when the patient requires new medication<ref> The patient may also request a repeat directly from the prescriber: see [[# | A new VV is needed when the patient’s medication stock is depleted or nearly used and the treatment may need to be continued (request repeat prescription). The patient either requests a repeat MVE from the pharmacist or has signed up in the past for proactive repeat MVE and a notification signal is generated by the repeat module of the AIS when the patient requires new medication<ref> The patient may also request a repeat directly from the prescriber: see [[#Process step: Informing the prescriber|paragraph 2.5.6]].</ref>. | ||
When the existing VV is not adequate, the pharmacist may communicate this request over the phone or send a digital VVV to the prescriber. The VVV may contain indications for the prescriber, such as urgency. The prescriber may approve the received proposal and alter it into a final VV. The prescriber informs the requestor via a AVVV (see also [[#Process step: Evaluating a | When the existing VV is not adequate, the pharmacist may communicate this request over the phone or send a digital VVV to the prescriber. The VVV may contain indications for the prescriber, such as urgency. The prescriber may approve the received proposal and alter it into a final VV. The prescriber informs the requestor via a AVVV (see also [[#Process step: Evaluating a pharmaceutical treatment|paragraph 2.2.4]] and subsequent paragraphs). | ||
===Process step: Creating an administration agreement=== | ===Process step: Creating an administration agreement=== | ||
| Regel 634: | Regel 634: | ||
The prescriber and the pharmacist as well as other health professionals and users all make use of an information system, respectively, an electronic prescribing system (EVS), a pharmacist information system (AIS, incl. ZAIS), a XIS and a PGO<ref> XIS is a generic term for a random (health care) information system. PHR=personal health records. </ref>. These information systems each have different system roles which enable the exchange of data between these information systems as part of the prescription process. | The prescriber and the pharmacist as well as other health professionals and users all make use of an information system, respectively, an electronic prescribing system (EVS), a pharmacist information system (AIS, incl. ZAIS), a XIS and a PGO<ref> XIS is a generic term for a random (health care) information system. PHR=personal health records. </ref>. These information systems each have different system roles which enable the exchange of data between these information systems as part of the prescription process. | ||
[[#Information systems and transactions|Chapter | [[#Information systems and transactions|Chapter 7]] includes an overview of all information systems, system roles, transactions, etc. The most important elements for the prescription process are included in the overview below. | ||
{{anchor|figuur 6}} | {{anchor|figuur 6}} | ||
| Regel 747: | Regel 747: | ||
==Process: medication use== | ==Process: medication use== | ||
This paragraph describes the process of medication use including registration of medication use by the patient or a health professional. The information recorded by the patient or health professional may be used, among other things, for medication verification by the health professional. During medication verification, medication use is established by the health professional | This paragraph describes the process of medication use including registration of medication use by the patient or a health professional. The information recorded by the patient or health professional may be used, among other things, for medication verification by the health professional. During medication verification, medication use is established by the health professional. | ||
===Current situation=== | ===Current situation=== | ||
| Regel 783: | Regel 783: | ||
====''Recording of medication use by health professionals''==== | ====''Recording of medication use by health professionals''==== | ||
During the process of medication verification ([[#Process: medication verification|paragraph 2.1]]) or evaluating a pharmaceutical treatment ([[#Process step: Evaluating a | During the process of medication verification ([[#Process: medication verification|paragraph 2.1]]) or evaluating a pharmaceutical treatment ([[#Process step: Evaluating a pharmaceutical treatment|2.2.4]]) the patient (or his informal caregiver) indicates, for example, that he does not use medication or uses it differently than was agreed. He may also indicate that he uses other medication as well (self-care products or foreign medication). The health professional can record these data as MGB. The data about medication use is recorded in addition to the primary medication data with the patient being recorded as the source of the information and the health professional as the author.<br> | ||
Instead of or in addition to recording the MGB, a prescriber may also choose to make a new or modified MA with the patient (in accordance with the process described in [[#Process step: | Instead of or in addition to recording the MGB, a prescriber may also choose to make a new or modified MA with the patient (in accordance with the process described in [[#Process step: Making a medication agreement|paragraph 2.2.5]]). This may occur when the prescriber sees reasons to adjust the agreement in response to the patient’s actual medication use. When the prescriber sees no reasons to adjust the agreement, he may choose to only record the medication use, perhaps with the remark that he has requested the patient to comply with the existing agreements. | ||
In case of deviations in MGB, a pharmacist may create a VMA for the prescriber (see [[#Process step: Informing the prescriber|paragraph 2.3.5]]) and/or advise the patient to inform the prescriber about the deviations. | In case of deviations in MGB, a pharmacist may create a VMA for the prescriber (see [[#Process step: Informing the prescriber|paragraph 2.3.5]]) and/or advise the patient to inform the prescriber about the deviations. | ||
| Regel 803: | Regel 803: | ||
===Information systems and transaction groups=== | ===Information systems and transaction groups=== | ||
The ''prescriber'' and the ''pharmacist'' as well as other ''health professionals'' and ''users'' all make use of an information system, respectively, an electronic prescribing system (EVS), a pharmacist information system (AIS, incl. ZAIS), a XIS and a PGO<ref>XIS is a generic term for a random (health care) information system. PHR=personal health records.</ref>. These information systems each have different system roles which enable the exchange of data between these information systems as part of the prescription process.<br> | The ''prescriber'' and the ''pharmacist'' as well as other ''health professionals'' and ''users'' all make use of an information system, respectively, an electronic prescribing system (EVS), a pharmacist information system (AIS, incl. ZAIS), a XIS and a PGO<ref>XIS is a generic term for a random (health care) information system. PHR=personal health records.</ref>. These information systems each have different system roles which enable the exchange of data between these information systems as part of the prescription process.<br> | ||
[[# | [[#Information systems and transactions|Chapter 7]] includes an overview of all information systems, system roles, transactions, etc. The most important elements for the prescription process are included in the overview below. | ||
{{anchor|figuur 8}} | {{anchor|figuur 8}} | ||
| Regel 825: | Regel 825: | ||
==After Hours General Practice clinics (HAP)== | ==After Hours General Practice clinics (HAP)== | ||
The after hours general practitioner (AHGP) works on behalf of the regular general practitioner (GP). The AHGP may (among other things) start, modify and discontinue MAs in accordance with the process described in [[#Process: medication verification|paragraph 2.1]]. If necessary, the AHGP will also create the corresponding VVs.<br> | The after hours general practitioner (AHGP) works on behalf of the regular general practitioner (GP). The AHGP may (among other things) start, modify and discontinue MAs in accordance with the process described in [[#Process: medication verification|paragraph 2.1]]. If necessary, the AHGP will also create the corresponding VVs.<br> | ||
The AHGP will inform the regular GP about the observation. Currently, it has been agreed that the AHGP does not act as a source of information for other health professionals of this patient. This means that the AHGP will not carry out the process step ‘(actively) making available’. Instead, the regular GP will make the relevant information available to the fellow health professionals. An exception may be made for a prescription created by the AHGP without a specified recipient, see [[#Prescription without specified recipient (ambulatory)|paragraph | The AHGP will inform the regular GP about the observation. Currently, it has been agreed that the AHGP does not act as a source of information for other health professionals of this patient. This means that the AHGP will not carry out the process step ‘(actively) making available’. Instead, the regular GP will make the relevant information available to the fellow health professionals. An exception may be made for a prescription created by the AHGP without a specified recipient, see [[#Prescription without specified recipient (ambulatory)|paragraph 9.1]].<br> | ||
In principle, the AHGP will follow the generic prescription process, except that a AHGP will carry out certain steps on behalf of the regular GP (‘delegated’). | In principle, the AHGP will follow the generic prescription process, except that a AHGP will carry out certain steps on behalf of the regular GP (‘delegated’). | ||
| Regel 836: | Regel 836: | ||
===Process=== | ===Process=== | ||
In the new situation, it will be possible to retrieve the available medication data and medication overviews. On this basis, medication verification, assessment of the medicamenteuze behandeling (as soon as possible) and medication administration (in accordance with [[#Medication process|Chapter 2]]) can be started. Medication dispense is carried out by public pharmacists on an outpatient basis and, in clinical practice, often by a contracted hospital pharmacy.<br> | In the new situation, it will be possible to retrieve the available medication data and medication overviews. On this basis, medication verification, assessment of the medicamenteuze behandeling (as soon as possible) and medication administration (in accordance with [[#Medication process|Chapter 2]]) can be started. Medication dispense is carried out by public pharmacists on an outpatient basis and, in clinical practice, often by a contracted hospital pharmacy.<br> | ||
As is the case with hospital discharge, medicamenteuze behandeling will be assessed at discharge from a mental health institution ([[#Process step: Evaluating a | As is the case with hospital discharge, medicamenteuze behandeling will be assessed at discharge from a mental health institution ([[#Process step: Evaluating a pharmaceutical treatment|paragraph 2.2.4]] and subsequent). Clinical medication is discontinued and, if necessary, new medication is prescribed or temporarily halted outpatient medication is resumed or permanently discontinued. The physician/psychiatrist focuses mainly on psychiatric medication; somatic medication usually remains unaffected.<br> | ||
Letting the psychiatric patient record his own medicatiegebruik may be experienced as undesirable control instead of it stimulating proper medication use.<br> | Letting the psychiatric patient record his own medicatiegebruik may be experienced as undesirable control instead of it stimulating proper medication use.<br> | ||
The medication data will be made available. | The medication data will be made available. | ||
| Regel 1.036: | Regel 1.036: | ||
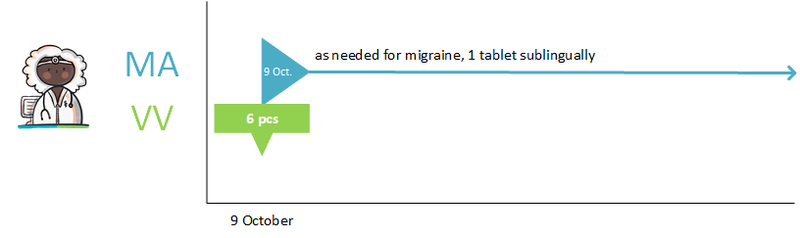
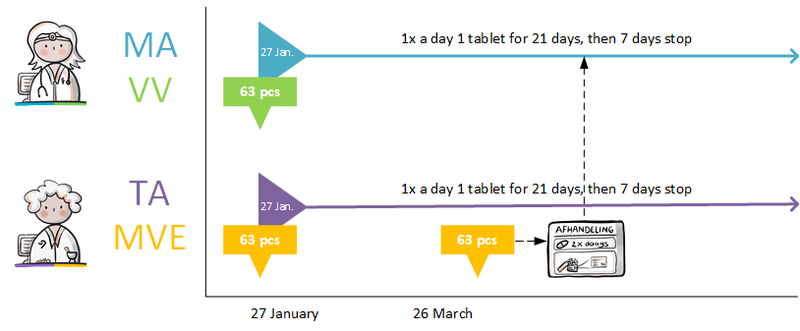
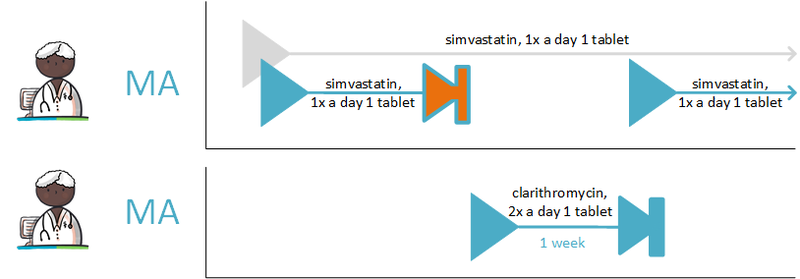
:''Simvastatin 40 mg; halt temporarily; for 1 week; reason: interaction'' | :''Simvastatin 40 mg; halt temporarily; for 1 week; reason: interaction'' | ||
:''Clarithromycin, 250 mg; 1 tablet 2x daily; for 1 week (and a corresponding verstrekkingsverzoek)'' | :''Clarithromycin, 250 mg; 1 tablet 2x daily; for 1 week (and a corresponding verstrekkingsverzoek)'' | ||
The pharmacist is actively informed by the general practitioner about the temporary halt of simvastatin. A new medicatieafspraak is also created with which simvastatin is resumed after one week. The pharmacist will also receive the medicatieafspraak and the corresponding verstrekkingsverzoek for clarithromycin. See also Temporarily halting and resuming medication in [[#Process step: Creating a | The pharmacist is actively informed by the general practitioner about the temporary halt of simvastatin. A new medicatieafspraak is also created with which simvastatin is resumed after one week. The pharmacist will also receive the medicatieafspraak and the corresponding verstrekkingsverzoek for clarithromycin. See also Temporarily halting and resuming medication in [[#Process step: Creating a medication agreement|paragraph 2.2.5]]. | ||
<br> | <br> | ||
| Regel 1.043: | Regel 1.043: | ||
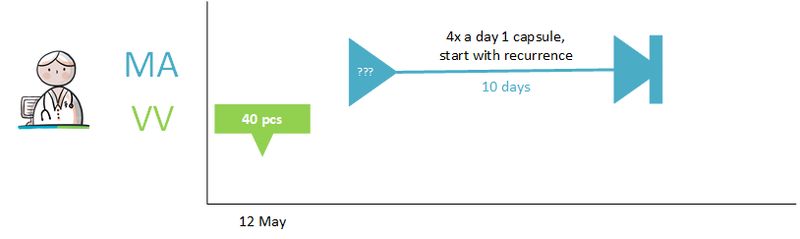
===Temporarily halting for an intervention=== | ===Temporarily halting for an intervention=== | ||
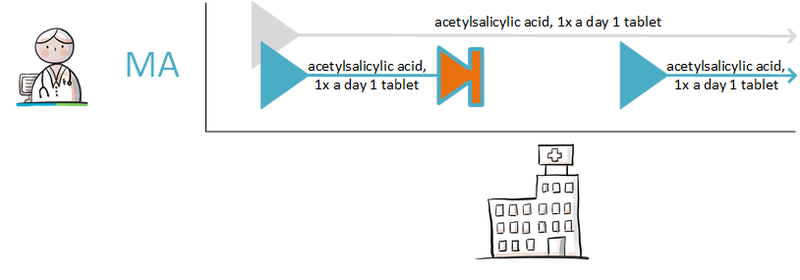
A 62-year-old man uses acetylsalicylic acid, 100 mg, 1 tablet 1x daily because of coronary disease. He has an intestinal polyp that must be removed. The general practitioner informs him that he should stop using acetylsalicylic acid three days before the intervention and resume the day after the intervention. The general practitioner records the temporary halt in a medicatieafspraak with an enddate 3 days before the intervention; stoptype ‘temporary’; reason for halting: ‘intervention’. In the (second) medicatieafspraak for presuming, 1 day after the intervention is used as the start date. The general practitioner actively informs the pharmacist and the gastroenterologist about the medicatieafspraken.<br> | A 62-year-old man uses acetylsalicylic acid, 100 mg, 1 tablet 1x daily because of coronary disease. He has an intestinal polyp that must be removed. The general practitioner informs him that he should stop using acetylsalicylic acid three days before the intervention and resume the day after the intervention. The general practitioner records the temporary halt in a medicatieafspraak with an enddate 3 days before the intervention; stoptype ‘temporary’; reason for halting: ‘intervention’. In the (second) medicatieafspraak for presuming, 1 day after the intervention is used as the start date. The general practitioner actively informs the pharmacist and the gastroenterologist about the medicatieafspraken.<br> | ||
When the date of the intervention is unknown or uncertain, the explanation will indicate that the medication should be halted three days before the intervention and resumed one day later. The duration of the temporary halt is then 4 days. See also Temporarily halting and resuming medication in [[#Process step: Creating a | When the date of the intervention is unknown or uncertain, the explanation will indicate that the medication should be halted three days before the intervention and resumed one day later. The duration of the temporary halt is then 4 days. See also Temporarily halting and resuming medication in [[#Process step: Creating a medication agreement|paragraph 2.2.5]] and Prior to admission in [[#Prior to admission|paragraaf 3.4.1]]. | ||
<br> | <br> | ||
| Regel 1.076: | Regel 1.076: | ||
===Transfer to another institution=== | ===Transfer to another institution=== | ||
In case of a transfer to another institution, the medicamenteuze behandeling is evaluated (see [[#Process step: Evaluating a | In case of a transfer to another institution, the medicamenteuze behandeling is evaluated (see [[#Process step: Evaluating a pharmaceutical treatment|Chapter 2.2.4]] and [[#Process step: Making a medication agreement|paragraph 2.2.5]]). Medication overview and medication data are (actively) made available. The new attending physician evaluates the medicamenteuze behandeling and determines the institutional medication. | ||
===Do not dispense before=== | ===Do not dispense before=== | ||
| Regel 1.085: | Regel 1.085: | ||
===Discontinuation of medication by third parties=== | ===Discontinuation of medication by third parties=== | ||
Medication can be discontinued by the prescriber himself (see [[#Discontinuing medication|paragraph 4.1.13]]) but also by another prescriber. For example, the specialist can end medicatieafspraken made by a general practitioner that are up to date according to the information system, but that turn out not to be, for example after medication verification. When a health professional discontinues medication, he creates a new stop-MA. The health professional cannot modify someone else’s medicatieafspraak, only create a stop-MA. He therefore actively informs the health professional of the original medicatieafspraak about this change. The health professional of the original medicatieafspraak modifies the end date in the existing medicatieafspraak. If required, this may also be automated by the information system. This prevents the second health professional from not having to produce data after a period of time because the production period has expired (also no stop-MA), while the health professional of the original medicatieafspraak continues to produce it for no reason (without an end date). | Medication can be discontinued by the prescriber himself (see [[#Discontinuing medication 2|paragraph 4.1.13]]) but also by another prescriber. For example, the specialist can end medicatieafspraken made by a general practitioner that are up to date according to the information system, but that turn out not to be, for example after medication verification. When a health professional discontinues medication, he creates a new stop-MA. The health professional cannot modify someone else’s medicatieafspraak, only create a stop-MA. He therefore actively informs the health professional of the original medicatieafspraak about this change. The health professional of the original medicatieafspraak modifies the end date in the existing medicatieafspraak. If required, this may also be automated by the information system. This prevents the second health professional from not having to produce data after a period of time because the production period has expired (also no stop-MA), while the health professional of the original medicatieafspraak continues to produce it for no reason (without an end date). | ||
<br> | <br> | ||
<br> | <br> | ||
| Regel 1.092: | Regel 1.092: | ||
===Two PRKs in a single medicamenteuze behandeling=== | ===Two PRKs in a single medicamenteuze behandeling=== | ||
In certain circumstances, the pharmacy is allowed to select a different PRK for the toedieningsafspraak than is indicated in the medicatieafspraak made by a prescriber. If for instance a failure of the prescriber’s information system would result in only the communication of the toedieningsafspraak, a subsequent prescriber will only have this toedieningsafspraak with the new PRK. The next prescriber will then probably assume that this PRK is also the PRK of the medicatieafspraak. A modification of the medicatieafspraak will then also result in a stop-MA (without referring to the original MA) and a new medicatieafspraak on the basis of this PRK. This means that there are now two medicatieafspraken with different PRKs under the same medicamenteuze behandeling. These two medicatieafspraken continue to exist. After any information system failure, (the information system of) the prescriber must check whether there have been changes and implement them, if necessary (see also [[#Discontinuation of medication by third parties|paragraph 4.1. | In certain circumstances, the pharmacy is allowed to select a different PRK for the toedieningsafspraak than is indicated in the medicatieafspraak made by a prescriber. If for instance a failure of the prescriber’s information system would result in only the communication of the toedieningsafspraak, a subsequent prescriber will only have this toedieningsafspraak with the new PRK. The next prescriber will then probably assume that this PRK is also the PRK of the medicatieafspraak. A modification of the medicatieafspraak will then also result in a stop-MA (without referring to the original MA) and a new medicatieafspraak on the basis of this PRK. This means that there are now two medicatieafspraken with different PRKs under the same medicamenteuze behandeling. These two medicatieafspraken continue to exist. After any information system failure, (the information system of) the prescriber must check whether there have been changes and implement them, if necessary (see also [[#Discontinuation of medication by third parties|paragraph 4.1.24]]). | ||
<br> | <br> | ||
[[Bestand:ENG_Voorschrijven_Twee PRKs onder een medicamenteuze behandeling.png|800px]] | [[Bestand:ENG_Voorschrijven_Twee PRKs onder een medicamenteuze behandeling.png|800px]] | ||
| Regel 1.098: | Regel 1.098: | ||
===Creating a medicatieafspraak after the fact=== | ===Creating a medicatieafspraak after the fact=== | ||
In emergency situations, for example, it may happen that the medicatieafspraak is only created after the medication has been dispensed or administered. This may lead to conflicting medicatieafspraken. This means that one or more medicatieafspraken must be discontinued in order to prevent the occurrence of parallel medicatieafspraken. The only exception for parallel medicatieafspraken is described in [[#Medicamenteuze behandeling|paragraph 1.3.3]]. | In emergency situations, for example, it may happen that the medicatieafspraak is only created after the medication has been dispensed or administered. This may lead to conflicting medicatieafspraken. This means that one or more medicatieafspraken must be discontinued in order to prevent the occurrence of parallel medicatieafspraken. The only exception for parallel medicatieafspraken is described in [[#'Medicamenteuze behandeling'|paragraph 1.3.3]]. | ||
===Single medication use=== | ===Single medication use=== | ||
| Regel 1.110: | Regel 1.110: | ||
===Inadvertently ‘outstanding’ medication or 'orphans'=== | ===Inadvertently ‘outstanding’ medication or 'orphans'=== | ||
In a transitional situation and in a situation where not every XIS is connected, medicatieafspraken for the same treatment may exist that have been created by different information systems under different medicamenteuze behandelings. These medicatieafspraken should ideally be combined in a single medicamenteuze behandeling. This particularly applies to medication with an open end date, as this medication may have been discontinued under a different medicamenteuze behandeling. The medicatieafspraak therefore remains open, resulting in continued medicatieverstrekking. This is a so-called ‘orphan’ (a building block that has been registered, but in which, at a certain point, the health professional no longer has an active role. However, his information system continues to provide the building block, even if it has already been discontinued elsewhere). For medication with an end date, this problem will solve itself. Therefore, it is not necessary to take any action. When medication verification or medication assessment reveals that the medication is inappropriately still ‘open’, a stop-MA is created under the same medicamenteuze behandeling. The original prescriber is actively informed about this stop-MA and enters an end date for the medicatieafspraak in his own information system. Depending on the supplier and the client’s wishes, setting this end date can be done automatically or through an intervention of the prescriber. See also [[#Discontinuation of medication by third parties|paragraph 4.1. | In a transitional situation and in a situation where not every XIS is connected, medicatieafspraken for the same treatment may exist that have been created by different information systems under different medicamenteuze behandelings. These medicatieafspraken should ideally be combined in a single medicamenteuze behandeling. This particularly applies to medication with an open end date, as this medication may have been discontinued under a different medicamenteuze behandeling. The medicatieafspraak therefore remains open, resulting in continued medicatieverstrekking. This is a so-called ‘orphan’ (a building block that has been registered, but in which, at a certain point, the health professional no longer has an active role. However, his information system continues to provide the building block, even if it has already been discontinued elsewhere). For medication with an end date, this problem will solve itself. Therefore, it is not necessary to take any action. When medication verification or medication assessment reveals that the medication is inappropriately still ‘open’, a stop-MA is created under the same medicamenteuze behandeling. The original prescriber is actively informed about this stop-MA and enters an end date for the medicatieafspraak in his own information system. Depending on the supplier and the client’s wishes, setting this end date can be done automatically or through an intervention of the prescriber. See also [[#Discontinuation of medication by third parties|paragraph 4.1.24]] and [[#Modification of someone else's medicatieafspraak|paragraph 4.1.37]]. | ||
<br> | <br> | ||
<br> | <br> | ||
| Regel 1.171: | Regel 1.171: | ||
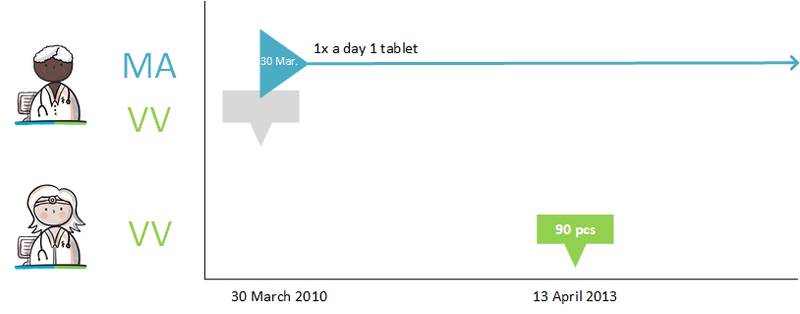
===Modification of someone else's medicatieafspraak === | ===Modification of someone else's medicatieafspraak === | ||
A patient takes 40 mg Lisinopril once a day because of hypertension. This medicatieafspraak was previously made by the GP with the patient. The patient is admitted to the | A patient takes 40 mg Lisinopril once a day because of hypertension. This medicatieafspraak was previously made by the GP with the patient. The patient is admitted to the A&E department because of a fall due to dizziness. The specialist notices hypotension and decides to reduce the dose of Lisinopril to 20 mg once a day. The specialist then stops the current medicatieafspraak and starts the new medicatieafspraak with a lower dose. With this he changes the medicationafspraak with the patient. The specialist informs the original prescriber (the general practitioner) about this with a stop medicatieafspraak and the new medicatieafspraak. | ||
The prescriber of the original medicatieafspraak adjusts the stop date in the original medicatieafspraak. If desired, this can also be automated by the information system. This prevents the second care provider no longer having to provide data over time because the delivery period has expired (including no stop MA) while the care provider of the original medication appointment continues to provide this incorrectly (without a stop date). | The prescriber of the original medicatieafspraak adjusts the stop date in the original medicatieafspraak. If desired, this can also be automated by the information system. This prevents the second care provider no longer having to provide data over time because the delivery period has expired (including no stop MA) while the care provider of the original medication appointment continues to provide this incorrectly (without a stop date). | ||
<br> | <br> | ||
[[Bestand:ENG_Modification of someone else's medicatieafspraak.PNG|800px]] | [[Bestand:ENG_Modification of someone else's medicatieafspraak.PNG|800px]] | ||
<br> | <br> | ||
===Setting up a variable dosing regimen (WDS) === | ===Setting up a variable dosing regimen (WDS) === | ||
The prescriber prescribes anticoagulants and states in the MA that the medication is used as recorded by the schedule of the thrombosis service (‘gebruik volgens schema trombosedienst). The prescriber sends a VV to the pharmacist and the patient is registered with the thrombosis service. In order to bridge the period until thrombosis care can take over, the prescriber creates a WDS for the first period. After registration, the thrombosis service creates a dosing regimen that overwrites or succeeds the previous regimen. From this moment on, the thrombosis service takes over creating the dosing regimen from the original prescriber. | The prescriber prescribes anticoagulants and states in the MA that the medication is used as recorded by the schedule of the thrombosis service (‘gebruik volgens schema trombosedienst). The prescriber sends a VV to the pharmacist and the patient is registered with the thrombosis service. In order to bridge the period until thrombosis care can take over, the prescriber creates a WDS for the first period. After registration, the thrombosis service creates a dosing regimen that overwrites or succeeds the previous regimen. From this moment on, the thrombosis service takes over creating the dosing regimen from the original prescriber. | ||
| Regel 1.343: | Regel 1.344: | ||
===Discontinuing medication in a GDS=== | ===Discontinuing medication in a GDS=== | ||
When medication is discontinued, the prescriber creates a discontinuation-medicatieafspraak. In accordance with the process, the pharmacist is informed about this stop-MA and can adjust the medicatieverstrekking accordingly. The pharmacist will create a staken toedieningsafspraak . See [[#Discontinuing medication|paragraphs 2.2.5.3]] and [[#Discontinuing | When medication is discontinued, the prescriber creates a discontinuation-medicatieafspraak. In accordance with the process, the pharmacist is informed about this stop-MA and can adjust the medicatieverstrekking accordingly. The pharmacist will create a staken toedieningsafspraak . See [[#Discontinuing medication|paragraphs 2.2.5.3]] and [[#Discontinuing an administration agreement|2.3.6.3]]. | ||
===GDS supplier supplies other commercial product=== | ===GDS supplier supplies other commercial product=== | ||
| Regel 1.677: | Regel 1.678: | ||
==Building block instantiations== | ==Building block instantiations== | ||
This section describes which instantiations of the building blocks belong to the medication overview. This concerns 'own' and 'other people's' building blocks ([[# | This section describes which instantiations of the building blocks belong to the medication overview. This concerns 'own' and 'other people's' building blocks ([[#After Hours General Practice clinics (HAP)|section 3.1]]) and then only the latest, relevant instantiations of this. | ||
===Own and other people=== | ===Own and other people=== | ||
| Regel 2.409: | Regel 2.410: | ||
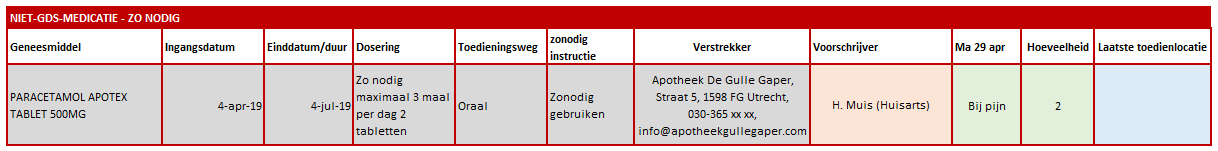
==Examples dosages== | ==Examples dosages== | ||
See [[mp:V9. | See [[mp:V9.2.0.0_Voorbeelden_doseringen|examples dosages 9 2.0.0]] (both functional and technical effect in HL7v3 CDA and in FHIR) | ||
==Medicatiegebruik: use indicator, according to agreement indicator, stop type, period of use and dosing instructions== | ==Medicatiegebruik: use indicator, according to agreement indicator, stop type, period of use and dosing instructions== | ||
| Regel 2.785: | Regel 2.786: | ||
==Qualification scripts== | ==Qualification scripts== | ||
[[mp: | [[mp:Vcurrent Kwalificatie|Qualification scripts]] | ||
=Attachment: Document history= | =Attachment: Document history= | ||
| Regel 2.900: | Regel 2.901: | ||
| style="background-color: white;vertical-align:top;"| 9 2.0.0 | | style="background-color: white;vertical-align:top;"| 9 2.0.0 | ||
| style="background-color: white;vertical-align:top;"| 05 April 2022 | | style="background-color: white;vertical-align:top;"| 05 April 2022 | ||
| style="background-color: white;vertical-align:top;"| all changes see: *[https://informatiestandaarden.nictiz.nl/wiki/mp:V2.0.0_releasenotes Releasenotes] | | style="background-color: white;vertical-align:top;"| all changes see: *[https://informatiestandaarden.nictiz.nl/wiki/mp:V2.0.0_releasenotes Releasenotes] | ||
|- | |||
| style="background-color: white;vertical-align:top;"| 9 2.0.0 | |||
| style="background-color: white;vertical-align:top;"| 08 April 2022 | |||
| style="background-color: white;vertical-align:top;"| broken internal links FO corrected [https://bits.nictiz.nl/browse/MP-607 BITS MP-607] | |||
|- | |||
| style="background-color: white;vertical-align:top;"| 9 2.0.0 | |||
| style="background-color: white;vertical-align:top;"| 14 April 2022 | |||
| style="background-color: white;vertical-align:top;"| Changed images of uce case 4.1.38, 4.1.39 and 4.1.40, they showed GDS instead of WDS [https://bits.nictiz.nl/browse/MP-534 BITS MP-534] | |||
|} | |} | ||
Huidige versie van 20 apr 2022 om 10:47
__NUMBEREDHEADINGS__
![]() This is the ENG version
This is the ENG version
![]() Klik hier voor de NL versie
Klik hier voor de NL versie
For an overview of relevant wiki pages for Medication Process see Landingspagina_Medicatieproces
Introduction
This document is the functional design for the Medication Process Information Standard (in Dutch: 'Informatiestandaard Medicatieproces'). It provides a general description as well as a description of specific practical situations. The recording and exchange of information is described for specific situations using actors (people, information systems) and transactions (which information is exchanged when).
Target groups for this document:
- Health professionals
- Information analysts and architects
- Software suppliers
Scope and vision
This document has been produced within the Medication process program. The Medication process program aims first to take away existing obstacles in the medication process, while taking into account current legislation and the possibility of obtaining tangible results in the foreseeable future.
One of the main obstacle entails the lack of insight in the actual medication use of patients. This is partly due to the fact that therapeutic and logistical information are often mixed, which results in the medication history becoming unclear. The following distinction between therapy and logistics exists:
- Therapy covers the medical side. This includes medication (and treatment) agreements, as well as the corresponding support and implementation. Therapeutic intention, (actual) medication use, self-medication and pharmacotherapy are also covered by the term ‘therapy’ as it is defined in this document.
- Logistics covers the physical flow of medicinal products, including requests, planning and dispense. This also includes medication supply and the medication use.
The program has taken into account current legislation and feasibility within the foreseeable future. The vision goes beyond the scope of the Medication process program and lays the foundations for a situation where a dispense request is no longer required. The ultimate objective is for prescribers to only have to concern themselves with the therapeutic side (which medicinal product, which strength, which dosage, when to start, etc.). It will no longer be necessary to create a dispense request. Instead, the prescriber will make medication agreements directly with the patient. Based on these medication agreements, the pharmacist will take care of the logistical process, eliminating the need for a dispense request altogether. Because of legislation this is not (yet) possible. The Medication process program does however take the first necessary step in the right direction.
Reading guide
The following paragraph introduces the main building blocks and the terminology used in this document. Detailed descriptions of the various processes (prescribe, dispense, administer, medication use) are given in Chapter 2. The purpose of the descriptions is to clarify how healthcare processes function in an ideal situation; which process steps are needed; which actors are participating; which information applies and which moments of exchange exist. The process descriptions follow a fixed format:
- Current situation
This paragraph describes the relevant differences between the current situation and the desired situation ('soll') in accordance with this information standard. Any obstacles will be described here. - Process description with the paragraphs:
- Precondition
The conditions that must be met before the process is started. - Trigger Event
The event that starts the process. - One or more process steps
Description of part of the process. - Post-condition
The conditions that are met after the process steps have been carried out. - Use cases
List of use cases associated with a specific subprocess. The use cases are detailed in Chapter 4. - Information sytems and transaction groups,
This paragraph describes the information systems, system roles, transactions and transaction groups related to the process steps. All information concerning information systems and transaction groups is also included in Chapter 7.
- Precondition
Chapter 3 describes a number of domain-specific interpretations of the medication process, for instance those of the thrombosis service and those related to service observation services in an ambulatory situation. Chapter 4 describes several use cases in more detail. The practical situations are derived from general medical practice in a large number of cases but are illustrative of similar situations in a different setting. The use cases are classified according to subprocess, as indicated in Chapter 2.
Chapter 5 describes how a medication profile can be constructed from the different building blocks. Chapter 7 includes an overview of all information systems, system roles, transactions and transaction groups. Guidelines for the functionality of the various information systems have been detailed in Chapter 8.
In this document, Dutch terminology is used for the medication building blocks: 'medicatieafspraak' (MA), 'verstrekkingsverzoek' (VV), 'toedieningsafspraak' (TA), 'medicatieverstrekking' (MVE), 'medicatietoediening' (MTD), 'medicatiegebruik' (MGB), 'medicatieverbruik' (MVB) (see Table 1 for the English translations). Dutch terminology for the medication building blocks is consistently used even in translated documents, as a translation may not refer to exactly the same entity.
Introduction of relevant terms
Therapeutic and logistical building blocks
The use cases include a description of the process and the data elements associated with it. Related data elements are grouped together in a Clinical Information Model (CIM) or Clinical Building Block (CBB) (in Dutch: 'zorginformatiebouwsteen' - zib). The dataset details the data elements of which these zibs consist; data elements may have been added to the zibs in keeping with the clinical context and care process. The data set includes the complete set of definitions of the data elements of the building blocks. The building blocks together with their data elements can be used in various scenarios for arranging/modelling healthcare applications or for defining interfaces for data exchange.
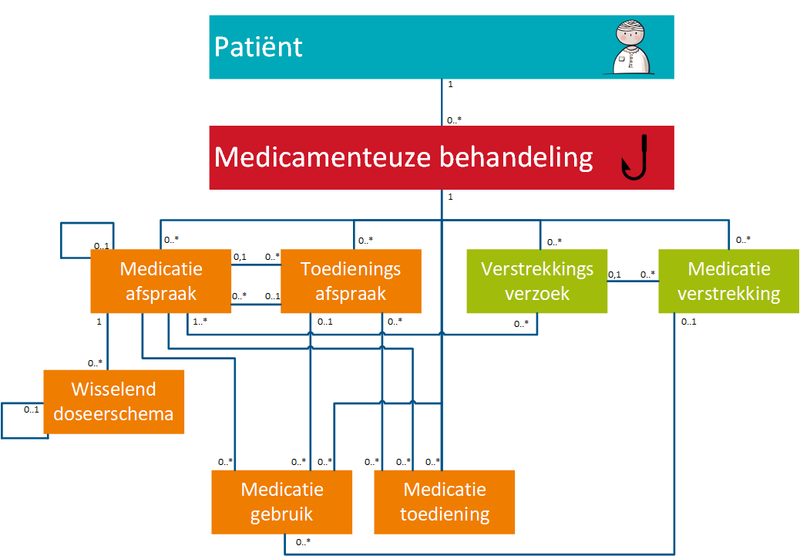
The different building blocks are shown in the figure below. They have been ordered according to process and subprocesses, and according to therapy versus logistics.
The table below provides a description of the building blocks. The four additional concepts ‘voorstel medicatieafspraak’ (therapeutic), 'antwoord voorstel medicatieafspraak' (therapeutic), ‘voorstel verstrekkingsverzoek’ (logistics) and ‘antwoord voorstel-verstrekkingsverzoek’ (logistics) are also described.
| Building blocks in Dutch | Abbr. in Dutch | Building blocks in English | Description |
|---|---|---|---|
| Medicatieafspraak | MA | Medication agreement | 'Medicatieafspraak' is the prescriber’s proposal for medication use with which the patient agrees. An agreement to discontinue medication is also a 'medicatieafspraak'[1]. |
| Wisselend doseerschema | WDS | Variable dosing regimen | 'Wisselend doseerschema' contains the dosing instruction as composed by an (external) prescriber. In the 'wisselend doseerschema' the element 'instructions for use' from the 'medicatieafspraak' is further specified. The 'wisselend doseerschema' can be adapted without changing the 'medicatieafspraak'. |
| Verstrekkingsverzoek | VV | Dispense request | 'Verstrekkingsverzoek' is the request from a prescriber to a pharmacist to supply the patient with one or more medicinal products in support of the current 'medicatieafspraak'/'medicatieafspraken'[2]. |
| Toedieningsafspraak | TA | Administration agreement | 'Toedieningsafspraak' contains the instructions for medication use (or administration) from the pharmacist to the patient (or his representative or administrator), adding to the 'medicatieafspraak'[3]. |
| Medicatieverstrekking | MVE | Medication dispense | 'Medicatieverstrekking' is the provision of a supply of medicinal product to the patient or his administrator or representative. |
| Medicatietoediening | MTD | Medication administration | 'Medicatietoediening' is the registration of the individual administrations of the medicinal product to the patient by the person who administers them (such as a nurse or the patient himself) in relation to the agreements made. |
| Medicatiegebruik | MGB | Medication use | 'Medicatiegebruik' is a statement about historical, current or intended use of a medicinal product[4]. |
| Medicatieverbruik | MVB | Medication consumption | 'Medicatieverbruik' is the logistical perspective on medication use. It describes how long a (partial) supply of medicinal products has lasted or will last for a patient[5]. |
| Voorstel medicatieafspraak | VMA | Proposed medication agreement | 'Voorstel medicatieafspraak' is a recommendation or request from the pharmacist, prescriber or the administer to the presciber of the MA about the agreed upon or to be agreed upon medication.The recommendation request may include discontinuing, starting or modifying medication. The patient is unable to send a VMA. |
| Antwoord voorstel medicatieafspraak | AVMA | Reply proposed medication agreement | 'Antwoord voorstel medicatieafspraak' is a replay from the prescriber to the 'voorstel medicatieafspraak'. |
| Voorstel verstrekkingsverzoek | VVV | Proposed dispense request | 'Voorstel verstrekkingsverzoek' is a proposal from the pharmacist to the prescriber to approve one or more 'medicatieverstrekking'/'medicatieverstrekkingen' in support of the current 'medicatieafspraak'/'medicatieafspraken'. This is comparable with the current situation of submitting the authorization form or combined prescription or submitting a repeat prescription for signing. The patient may also submit a 'voorstel verstrekkingsverzoek' to the prescriber. |
| Antwoord voorstel‐verstrekkingsverzoek | AVVV | Reply proposed dispense request | 'Antwoord voorstel-verstrekkingsverzoek' is a reply from the prescriber to the 'voorstel verstrekkingsverzoek'. |
Table 1 Building blocks – description
Medication overview
See Chapter 5 for more information about these overviews, the applicable building blocks and how a medication profile/current overview can be compiled.
'Medicamenteuze behandeling'
The different medication building blocks represent steps in the medication process, from prescribing a medicinal product (MA and/or VV), followed by dispensing it (TA and/or MVE) up to and including administering and using the medicinal product. The model is designed in such a way that therapeutic building blocks and logistical building blocks are separated from each other.
Scope
In order to be able to give a name to the interdependence of the medication building blocks, the concept of ‘pharmaceutical treatment’ (in Dutch: 'medicamenteuze behandeling' - MBH) is introduced.
- 'Medicamenteuze behandeling' is a technical concept in the information standard. Its purpose is:
- To unambiguously identify the set of interdependent medication building blocks, and
- To apply rules to it to unambiguously determine the present situation.
- To unambiguously identify the set of interdependent medication building blocks, and
The functional application of the concept of 'medicamenteuze behandeling' is as follows:
- Medication (or 'medicamenteuze behandeling') is started by creating a first MA as part of a new 'medicamenteuze behandeling'.
- Medication (or 'medicamenteuze behandeling') is discontinued by creating a new MA within the same 'medicamenteuze behandeling' (stop-MA).
- Medication (or 'medicamenteuze behandeling') is modified by:
- Discontinuing the existing MA and
- Creating a new changed MA as part of the same 'medicamenteuze behandeling'. The starting date of this new MA may also be in the future.
Prescribing a new medicinal product always results in a new MA. An MA is always related to a single 'medicamenteuze behandeling'. For the time being, the PRK level (Prescription Code from the G-standard) of the medicinal product determines whether the MA belongs to a new or an existing 'medicamenteuze behandeling'. This may be extended to the SNK level (Substance Name Code from the G-standard) in the future, which would mean that changes in strength or between medicinal products from the same group no longer lead to a new 'medicamenteuze behandeling'. A detailed description can be found in paragraph 2.2.5 Process step: Making a medicatieafspraak.
Exceptions:
- Products without PRK (a non-medicine such as crutches or bandages). In this case the HPK level (Trade Product Code from the G-standard) determines if the MA will lead to a new 'medicamenteuze behandeling'.
- Medication without PRK (magistrals often consist of several substances that are not covered by the same PRK, these substances are included separately as ingredients in the MA). Every magisterial or adaptation to it falls under a 'medicamenteuze behandeling'.
- Own articles without PRK (articles listed in the internal information system under 90 million numbers stored, such as half tablets, commonly used magistrals). Any item or adaptation to this falls under a 'medicamenteuze behandeling'.
- Intravenous (IV) therapy (still be selected).
Examples
Five examples illustrate the scope of a 'medicamenteuze behandeling':
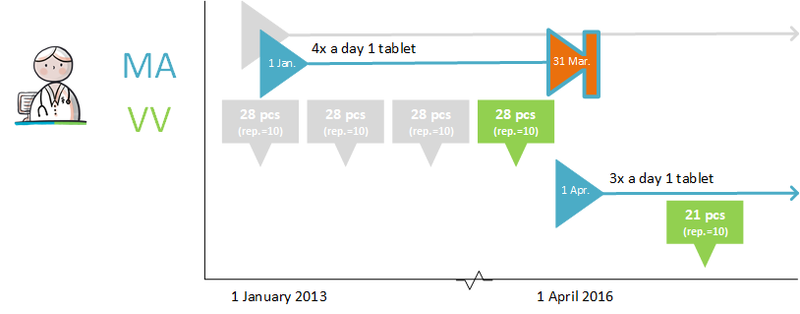
- Diazepam, 5 mg, 1 tablet 4x daily is changed to diazepam, 5 mg, 1 tablet 3x daily. The PRK level of both products is the same, they are both part of the same 'medicamenteuze behandeling'.
- Paroxetin tablet, 10 mg, 1 tablet 1x daily, is changed to paroxetin tablet, 20 mg, 1 tablet 1x daily. This is a modification of an MA with two different medicinal products at the PRK level. This change requires that the first 'medicamenteuze behandeling' is discontinued and a new 'medicamenteuze behandeling' is started.
- A gastroprotective drugs has been agreed upon in a treatment with prednisone: prednisone and gastroprotective drugs are two different medicinal products, which are used parallel to each other and their use can be modified and discontinued independently of each other. This means they are not part of the same 'medicamenteuze behandeling'.
- Switching from a beta blocker to an ACE inhibitor means a new PRK and this is achieved by discontinuing the 'medicamenteuze behandeling' of the beta blocker and starting a new 'medicamenteuze behandeling' for the ACE inhibitor.
- When there is no PRK and the composition of the medicinal products in the MA changes (any change in the ingredients), the existing 'medicamenteuze behandeling' is discontinued and a new 'medicamenteuze behandeling' is started. This applies, for example, to extemporaneous preparations, drips and proprietary products.
Creation of a 'medicamenteuze behandeling'
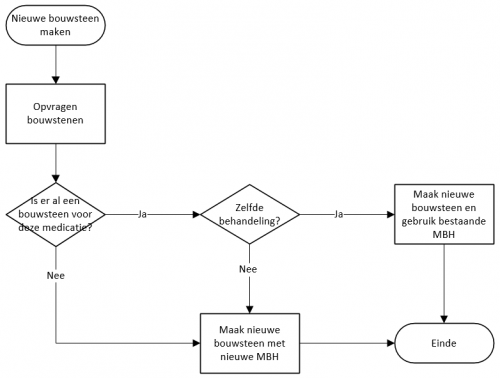
A schematic overview of how a 'medicamenteuze behandeling' (MBH) is set up can be seen in the figure below. When a new building block (MA, TA, VV, MVE, MTD or MGB) is created, a check is first performed to determine whether this is a new medication or if there already is a current building block with the same product. This applies to all building blocks, both proprietary or third parties’. In most information systems, the user of the information system will indicate that he wants to change one of the existing medication building blocks or wants to introduce new medication. In that case, it is easy to find out whether there already is a 'medicamenteuze behandeling' that includes the building block.
- If there is no existing building block for this medication, a new building block with a new 'medicamenteuze behandeling' is created.
- If there is an existing building block with a 'medicamenteuze behandeling', the user of the information system is asked whether the new building block and the existing building block belong to the same treatment. When this is the case, the same 'medicamenteuze behandeling' will be used. When the building blocks do not belong to the same treatment, a new 'medicamenteuze behandeling' will be created.
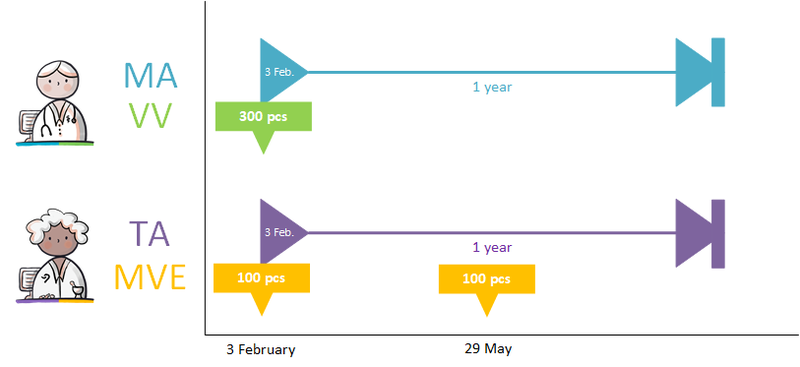
Parallel occurrence of 'medicatieafspraken'
Within a 'medicamenteuze behandeling', several MAs may be active simultaneously. These are all MAs that are valid ('current') at this time or that will become valid in the future. In principle, only one MA is valid at any time in a 'medicamenteuze behandeling'. However, there are a number of situations where parallel MAs are conceivable:
- The same medicinal product, but a different strength, where the total strength should essentially be prescribed in one agreement.
- Related (different) medicinal products that are given together, but that should be considered as a whole when evaluating treatment.
- Technical omissions in information systems. For example, in the case of complicated dosing schedules or combination drips.
Situation 1 can be resolved by prescribing at a higher level (SNK). G-standard/Z-Index is working on this, but it is not yet possible. Until that time, 1 or more products can be combined in the same MA by entering the products as ingredients. This is comparable to extemporaneous preparations. However, the instructions for use for all these products must be identical.[6]
Situation 2 is solved in different ways in different information systems, each with their own grouping mechanisms. It concerns the correlation between different medicamenteuze behandelingmedicamenteuze behandelings. The information standard does not provide a universal grouping mechanism.
Situation 3 is the only situation in which parallel medicatieafspraken are permitted under one medicamenteuze behandelingmedicamenteuze behandeling. Complex phasing-in and phasing-out schedules and combination drips may be included in one medicatieafspraak, but not all information systems support this. For those information systems, it is permitted to create parallel medicatieafspraken within a single medicamenteuze behandelingmedicamenteuze behandeling.
Correlation between building blocks and 'medicamenteuze behandeling'
The figure below shows the correlations between building blocks and the MBH. The relations between building blocks and the MBH as well as the relations between the building blocks themselves are described as follows:
- Building blocks belong to a single MBH. An MBH includes at least one MA and may include zero or more of the following building blocks VV, WDS, TA, MVE, MGB and MTD. Unless, for example, self-medication has been recorded with MGB or in case a paper prescription has been submitted, a drug treatment can exist without an MA, but with MGB or TA. A MBH will never cease to exist, but it may no longer be effective when there are no current building blocks linked to it. A VMA, AVMA, VVV and AVVV are not yet part of a MBH as these are still a draft/proposal that may or may not lead to a final MA or VV linked to an MBH. A VMA may lead to zero (if the recommendation is not followed), one or more MAs and a VVV may lead to zero (if the proposal is not honoured), one or more VVs.
- An MBH may also only have a stop-MA in addition to, for example, an MGB building block. For example, in the event that a health professional asks a patient to stop using free available medicine (self-care medication or over-the-counter (OTC) medication). The health professional records the use of the self-care medication in an MGB building block and discontinues the use by creating a stop-MA (stop-MA belonging to the same MBH).
- An MA may refer to the previous MA or a TA or MGB on which it is based. This may also be an MA, TA or MGB that belongs to another MBH. It is possible that no digital MA is available (e.g. paper prescription paragraph 4.1.17). This MA must then be created. This MA may refer to the TA or MGB. A pharmacist is never the source of an MA but he may have a copy.
- In principle, only one MA is valid at any one time in an MBH. Only when there are technical omissions in information systems, for example in case of complicated dosing schedules or combination drips, parallel MAs are allowed (see also the previous section).
- An MA is supported by zero (if there is still enough supply or if no medication dispense is needed), one or more (when there is, for example, continuous medication) VVs.
- A VV is based on the current MA and any existing corresponding TA in an MBH. There may be several.
- A VV refers to one or more MAs (for example, in the case of an interim dosage increase, a VV can be made that replenishes the supply for the existing MA and also starts the supply for the future MA).
- A VV may result in zero (for example when the patient does not pick up the medication) to several MVEs.
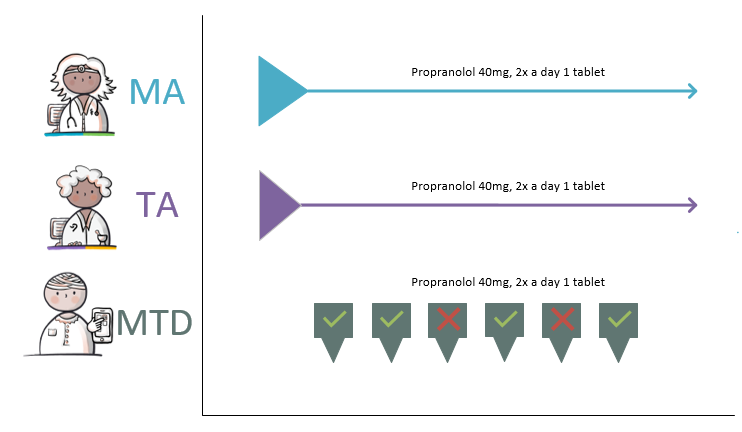
- A MA may result in zero, one or multiple WDS's.
- Multiple (possibly parallel) TAs may be based on the same MA (for example, when a pharmacist switches to a different commercial product or when the medicinal product is supplied as two or more medicinal products with different strengths, with the total strength remaining the same). When a paper prescription is submitted and the MA and the VV are not available in digital form, there is a TA without an MA.
- An MA does not always have to lead to a TA, for example when no VV is required with a short use MA, when the patient still has sufficient stock.
- A TA is supported by zero (when there is enough supply), one or more MVEs.
- An MVE is based on a MA (and TA) and, in an ambulatory situation, on a VV. The exception are over-the-counter (OTC/self-medication) sales for the purpose of self-care medication: these have no MAs and no VVs. Self-care medication provided by the pharmacist may be recorded by that pharmacist as a TA with MVE, or as MGB by a random health professional or by the patient himself.
- An MVE may support multiple TAs.
- An MA or a TA may be followed by a new MA or TA. This may be the case when existing medication is changed (modification of MA and/or TA) or when MGB is discontinued (stop-MA/TA).
Informing and (actively) making available
Chapter 2 describes the medication process. This includes process steps labelled ‘Inform’ or ‘(actively) make available’. The table below includes a detailed description of the reason for this. Chapter 2 further elaborates on situations in which information is actively sent or made available.
| Term | Toelichting |
|---|---|
| Inform | ‘Inform’ means sending an order or request addressed to another actor. This is a so-called ‘push’ of information: the actor sends the information specifically to recipient. This may be by means of
|
| (Actively) make available | The health professional (actively) makes information available to another actor. This process step concerns:
Data are always made available. Additionally, the health professional has the option to specifically inform fellow health professionals and/or the patient at any time during the process. In consultation with the patient, data can be sent to a health professional of his choice (inform). |
Table 2 Informing versus (actively) making available
The technical mechanism (‘pull’, ‘publish and subscribe’, ‘push’, etc.) used to (actively) make data available, is infrastructure-dependent and is therefore not detailed in this document.
The Royal Dutch Medical Association (KNMG) describes in its publication 'Van wet naar praktijk: implementatie van de WGBO Deel 4. Toegang tot patiëntengegevens' [From law to practice: implementation of the Dutch Medical Treatment Contracts Act Part 4. Access to patient data] when there is implicit consent and explicit consent. The exchange described in this information standard is subject to current legal frameworks and guidelines concerning consent and opt-in and these are therefore not explicitly described.
Legend/Explanation
A manual for this Nictiz wiki documentation can be found at:
http://informatiestandaarden.nictiz.nl/wiki/Handleiding_Wiki_documentatie
It also includes a legend for the various figures that appear in this document.
Medication process
This chapter describes the medication process in relation to the building blocks for first-line, second-line and third-line health care. The process is fundamentally the same in each case. The main difference is which pharmacy supplies the medication: a community pharmacy (including an outpatient pharmacy) or a hospital pharmacy. Another difference is that in an ambulatory setting a VV is required for the supply of medicinal products. This is not required in a hospital setting: the (hospital) pharmacist ensures that the medicinal products are available as long as the MA continues.
The medication process is a cyclical process consisting of prescribing, dispensing, administering and using medication. The process starts when the patient/client visits a health professional/healthcare provider (general practitioner, hospital or other institution) for a treatment with a medicinal product and ends when the medication is no longer needed. The process is depicted in Figure 4. The yellow bar indicates the medication verification process, green prescription, purple dispensing, and orange administration and medication use. The blue bar indicates receipt or retrieval of data made available. This may occur in any of the subprocesses and is described in more detail in the continuation of this chapter for the relevant subprocesses. The following paragraphs describe the medication verification, prescription, dispensing, administration and medication use processes.
Process: medication verification
Prior to the prescription process, the patient’s actual medication use is determined. This is done[7]:
- In the GP practice by the general practitioner during a consultation,
- At the GP service, A&E department or mental health crisis service by the triage specialist or the treating physician, as soon as possible, upon arrival or admission,
- In case of clinical or day admission at a hospital or other institutions by for example the nursing staff, pharmacy assistent or outpatient/hospital pharmacist,
- In case of outpatient consultation by for example nursing staff, doctors’ assistant or the treating physician.
Current situation
- In the current situation, patients or family/informal caregivers are asked which medication they are using. The patient is sometimes unable to answer this. Family/informal caregivers (if known) are also often unable to answer this. If this is the case, the physician will contact the general practitioner or the pharmacist to find out the medication. This is difficult outside office hours and during weekends.
Precondition
The patient comes in for a consultation/an outpatient consultation or is admitted (in the future).
Trigger event
- Outpatient setting: consultation of and/or prescription to outpatients and patients residing in another healthcare institution[8]. In this case, medication verification often occurs during treatment assessment (see paragraph 2.2.4).
- Clinical setting: preparation of patient admission.
Process
The health professional collects the medication data from various sources, which may include:
- Patient’s own story,
- Dispense overviews from pharmacies,
- Digitally available medication data from healthcare providers or personal health records (PGO),
- Medication brought in by the patient,
- If necessary, information by telephone from the patient’s own pharmacist or general practitioner.
The health professional verifies the medication together with the patient and records the verified medication as MGB, incl. self medication. This results in an updated medication profile; see also paragraph 5.6.
In practice, medication verification will lead to recording of MGB, only when it proves clinically relevant followed by updating the medication profile, particularly upon admission and discharge. The medication overview and recorded data on MGB are made available to fellow health professionals and the patient, so that they can access the data. The medication overview may also be sent to a specific health professional.
Post-condition
The MGB is recorded and the resulting medication overview is made available if created. Medication data (MGB) are made available.
Information systems and transaction groups
Chapter 7 includes an overview of all information systems, system roles, transactions, etc. Those most important for the medication verification process are included in the overview below.
Process: prescribe
This paragraph describes the prescription process. This includes all prescribers, such as general practitioners, specialists, other physicians and specialist nurse prescribers. The prescription process consists of an evaluation of existing pharmaceutical treatment, if any. If necessary, an medication agreement is created and, only in an ambulatory situation, possibly a medication dispense request. Finally, the recorded data are (actively) made available.
Current situation
The following deviations from the desired situation that are currently observed are:
- The logistical process often determines whether information is recorded (and certainly if it is communicated). Changes in medication or discontinuation are insufficiently recorded and/or communicated, resulting in, among other things, inaccurate monitoring, incorrect use and incorrect medication profiles.
The pharmacotherapeutic policy should be leading, not the logistical process as is currently the case.
- Since the therapeutic intention is not communicated to the pharmacist, it is not possible to deduce from the available data whether a request for a repeat prescription falls within that therapeutic intention. Because of this, use may be erroneously resumed or continued.
- If a change is not communicated, a request for a repeat prescription (through the pharmacist) may be based on outdated instructions for use. This can easily lead to errors.
- In an outpatient setting, medication agreements and/or medication dispense requests are usually not (electronically) sent to the pharmacist.
Precondition
There is a certain reason why a prescriber wants to start or evaluate/review a (pharamceutical) treatment.
Trigger event
The trigger event for the process is the start of a new MBH, the evaluation of an ongoing treatment, receipt of a VVV or VMA, receipt from a pharmacist of a prescription to be processed, or patient admission to or patient discharge from an institution.
Process step: Evaluating a pharmaceutical treatment
In order to evaluate treatment, an up-to-date overview of medication data is required. The medical file from the health professional is, where possible and if necessary, updated with data from external sources. In addition, the patient may be asked which medicinal products he is currently using. This medication use can be recorded by the health professional. If desired, a more extensive medication verification can be carried out (see paragraph 2.1).
The treating physician[9] evaluates the (pharmaceutical) treatment and decides to:
- start a new MBH by creating an initial MA and/or
- continue, discontinue, temporarily halt or modify an existing MA (1 or more)[10] and/or
- correct/cancel an existing MA and/or
- approve a VMA or a VVV (including a reply via the AVVV)
These situations are further explained in the following paragraph. See also paragraph 1.3.3 for more information on the concept of 'MBH'. A new medication overview may be created to conclude the evaluation and the resulting new agreements and dispense requests (see paragraph 5.6).
Process step: Making a medication agreement
When creating an MA, the following principle applies: each change is recorded in a new MA. Technically this means that the existing MA is terminated by entering an end date for the period of use and that a new MA is created with the desired changes[11].
An MA can also be created to begin at a point in the future. These MAs will receive a period of use with future starting date which is later than the date of the agreement itself. Any prior MA will end just before the starting date/time of the future one. In the period of use, only one effective date can be indicated (without duration or end date), this is the case with continuous medication. To avoid confusion between 'to/until/till' and 'up to and including', specifying the time is mandatory when entering an end date. In case of an 'up to and including' date (in case of an entire day), the time 23:59:59 applies.
Before the MA is made (actively) available, medication monitoring will occur in accordance with current guidelines. This is a part of this process step.
The next paragraphs describe the different situations in which an MA is created, i.e. initial medication agreement, continuing medication, discontinuing medication, temporarily halting medication or correcting/canceling a agreed medication. Information about 'MBH' is assumed to be known (see paragraph 1.3.3).
New medication agreement
A new MA is created at the start or modification of an MBH. When a new MBH is started, the prescriber should consider whether an existing MBH should be discontinued. The description in paragraph 1.3.3 is based on the most common process from prescription to administering or using. In a transitional situation or in the absence of digital data, it is also technically possible that an MBH could start with a TA, for example. This might occur for instance when a pharmacist has not received the MA with the corresponding MBH in digital form. The pharmacist will consequently start a new MBH when the TA is created. This may also be the case for any other building block. A patient can, for example, start an MBH by recording MGB, without having the original MBH.
Continuing medication
In a number of cases the therapeutic intention of the prescriber remains the same and the MA does not have to be modified. For instance:
- In an ambulatory situation when, for a repeat prescription, only a new VV is needed, or
- At admission to an institution where the home medication continues to be used, whether or not in combination with self-medication.
In both of these cases, the existing MA will not be adjusted. Should there be a change in PRK, e.g. at admission or discharge, the existing MBH will be discontinued by creating a stop-MA (see paragraph 2.2.5.3) and a new MBH is started (see paragraph 2.2.5.1).
Discontinuing medication
Medication is discontinued by creating a new MA (stop-MA) within the same MBH. The reason for discontinuation is recorded in this MA. The medication may be discontinued immediately or in the future. The new MA (stop-MA) is a copy of the existing MA with:
- The period of use as end date on which the MA ends (may also be in the future),
- An end date which has to be included in the text description of the instruction for use as well,
- Its own author,
- Its own agreement date,
- Stop type 'permanent',
- Reference to the specific MA that will be changed (future MAs will remain in place). It is not possible to create a stop-MA without referring to the MA that needs to be stopped except when the MA is not available. If there are only TA('s) or MGB('s) available in the MBH then a stop-MA has to be able to end these building blocks without referring to a MA,
- Its own reason for the MA to be discontinued (this does not apply to a stop-MA made as part of a modification. In that case, no reason is given).
For an MA in which an end date is immediately agreed upon, e.g. in the case of a course of treatment, no additional stop-MA is needed. When an MA with an end date in the future is extended, it will be considered as a normal change (see paragraph 2.2.5.5). A stop-MA always has the ‘permanent’ stop type, even if it is a stop-MA resulting from a change. In case of a change, the stop-MA is followed by a new MA. A stop-MA resulting from a change is not always relevant for end users. A stop-MA as a result of a change is also referred to as a technical stop-MA. The user interface must adequately support this. A prescriber will be less interested than a pharmacist who may need to adjust his logistical process because of the change.
Temporarily halting and resuming medication
Temporarily halting medication is the discontinuation of medication for a known or unknown period of time. Medication may be halted immediately or in the future. When medication use is temporarily halted, the medication still remains relevant for monitoring because the medication may be resumed in the future. Temporary substitution with another medicinal product is not considered an interruption but rather a discontinuation of the original medication and the start of a new pharmaceutical treatment with the substitute. Temporary halting medication is covered by two medication agreements[12]: a MA (stop-MA) is created to stop medication use in accordance with guidelines for a stop-MA (see previous paragraph) and a new MA is created for resuming the medication (including the reason for this, if any). All MAs are part of the same MBH. The reason for the interruption is recorded in the stop-MA. The stop type for the stop-MA is 'temporary’.
Changing medication
Medication modifications may be related to:
- a) Dosage,
- b) Strength of the medicinal product,
- c) Method of administration,
- d) Duration of treatment (such as an extension of the therapy).
Switching to a completely different medicinal product is technically a switch to a different MBH (see also paragraph 1.3.3). In that case, the physician will discontinue the existing pharamceutical treatment (see paragraph 2.2.5.3) and start a new one (see paragraph 2.2.5.1). If the PRK stays the same, changes will be recorded under the same MBH. When a modification needs to be made, a technical stop-MA is created (see paragraph 2.2.5.3) and a new MA is made with the desired change. The appointment date of the technical stop-MA and the new MA must be always be the same. The reason for the modification is included in the new MA with a reference to the original MA, if possible. Modifications may take effect immediately or in the future. A technical stop-MA and the related new MA are made available simultaneously. In case of renewal of an MA of which the duration has already expired or the end date has already expired, then this is not seen as a change (a stop-MA on the already automatically stopped MA is unnecessary). In this case, a new MA under the same MBH can be made.
Correcting/canceling a medication agreement[13]
This paragraph describes correcting or canceling an MA when the prescriber has made an error. The error may have been discovered by the prescriber himself or by a fellow health professional. For example, a physician who makes a typing error in the dosage of an MA: 10 inhalations, 2x daily, instead of 1 inhalation, 2x daily. If the MA has not yet been shared with other health professionals, the prescriber can personally correct that MA or delete (cancel) it from his own information system. If the MA has already been shared with other health professionals, the prescriber will mark the incorrect MA as ‘incorrect registration’ and he will then create a new MA under the same MBH with the correct information. The prescriber will actively inform the pharmacist and any fellow health professionals about both the cancelled and new MA and will make both available (see paragraph 2.2.10).
Stopping a prospective medication agreement
This only concerns MA's that have their start date at some point in the future. For whatever reason a prescriber wants to end this prospective MA. The prescriber will not create a stop-MA in this scenario but cancels the MA instead. The prescriber cancels the MA by using the ‘canceled indicator’ field. The prescriber can only use the ‘canceled indicator’ when the start date of the MA is in the future. If the prescriber wants to end the prospective MA he/she changes the status of the MA to canceled with the indicator.
When the prescriber is not the initial creator of the original prospective MA the process works as follows. The prescriber creates a new MA with the canceled indicator activated. The ‘canceled MA’ will be sent to the original prescriber of the MA. The original prescriber will then cancel the original MA as well.
Process step: Making a variable dosing regimen
When a prescriber prescribes medication combined with a variable dosign regimen (WDS), the dosing of the medication can be adjusted by a (different) prescriber without having to adapt the medication agreement (MA). At the moment the variable dosing regimen is used for anticoagulants. When prescribing anticoagulants, the prescriber determines the therapeutic INR-range (International Normalized Ratio, a measure of blood clotting time), within which the treatment should take place.
The thrombosis service is responsible for drafting the viariable dosing regimen. The dosing regimen is composed by a prescriber (usually a thrombosis physician) in the building block variable dosing regimen (Wisselend doseerschema, WDS). The prescriber who made the medication agreement remains responsible for the dispense requests (VV). The thrombosis physician composes the WDS withing the agreed upon INR-range, the dosing regimen is usually based on a specific, measured INR-value.
Setting up a variable dosing regimen
The prescriber prescribes anticoagulants with a medication agreement (MA) and indicates:
- The medication (PRK) that is being prescribed to the patient. The medication in the variable dosing regimen is always the same as the medication in the MA.
- That for this medication a variable dosing regimen applies (this is added in the additional instructions). This also means there will be no dosage added in the medication agreement.
- The INR-range within which the treatment should take place. This information is included in a comment.
In order to bridge the period until the thrombosis service is involved and ready to take over the treatment, the original prescriber sets up a WDS for this first period (usually between 4 and 7 days). Usually a check-up date is agreed upon after the registration of the patient at the thrombosis service. During this check up the INR-value is measured. Based on the measured value and/ or the professional assessment of the thrombosis physician, a dosing regimen is composed that either changes of succeeds the previous WDS. From this moment on, the thrombosis service takes over the composition of the dosing regiment from the original prescriber.
INR-value with the WDS
The variable dosing regimen is often based on an INR-value. For other parties involved in the care for the patient, it’s important to be able to deduce the INR-value on which the WDS was based. That is the reason that the corresponding INR-value will be made available within the WDS. The INR-value is included in a comment within the WDS.
Changing a variable dosing regimen
When the variable dosing regimen (WDS) is being used up until the stop date of the WDS, it can be replaced by a new WDS that succeeds it. The new WDS has a relation to the MA and the previous WDS. Furthermore, it is possible to adjust the WDS before the stop date is reached. In that case, the information system stops the previous WDS, using a technical stop-WDS that is not visible to the user. The new WDS follows and has a reason for change and a relation to the MA and the previous WDS. All changes related to the dosing regimen can be included in the WDS. Changes that concert the further treatment policy (e.g. the prescribed medication, the route of administration of the agreed upon INR-range) are to be made in the MA.
Stopping a variable dosing regimen
There are various reasons that can cause the need to (temporarily) stop the use of anticoagulants. For example, in the event of a procedure of because there is temporary co-medication. Two kinds of situations can be distinguished. Depending on the situation and the assessment of the thrombosis physician one of twee methods is chosen:
- (temporary) adjusting the policy: The thrombosis physician can choose to temporarily adjust the dose for the anticoagulants to 0. For example, in the event of a planned procedure. In this case, the MA (and therefore the treatment with anticoagulants) and the TA will continue, but the dosage in the WDS is temporarily adjusted to 0. The MA will still be shown on the medication overview under ‘current medication’. In the event of such a temporary adjustment, only the WDS is changed. It is advised to include a reason for this change in the new WDS.
- (temporary) stopping the anticoagulants: The thrombosis physician (or another prescriber) can also choose to (temporarily) stop the treatment with anticoagulants. This means that the patient should not to take any anticoagulants anymore. In this case the thombosis physician (or another prescriber) creates a stop-MA (or sends a proposed MA to the original prescriber). Stopping the MA, also stops the underlying therapeutic building blocks (TA, WDS). The anticoagulant will be shown on the medication overview under ‘recently stopped medication’.
If, after a period of time, there is a need to restart the anticoagulant, the prescriber can do this by creating a new MA. The new MA can be created by the original prescriber, the thrombosis physician could also send a proposed MA. In many cases the thrombosis service would no longer be involved. And the MA is created by the original prescriber.
Process step: Creating a medication dispense request
A VV (besides an MA) only applies in an ambulatory situation. A VV may be made when the medication supply of the patient needs to be replenished. This does not have to coincide with an MA. At the start of a MBH for which the patient still has a sufficient supply at home from a previous agreement, a VV is not needed. When the dosage is reduced, the patient may also have a sufficient supply. In the case of a medicinal product that is used for a prolonged period of time (e.g. an antihypertensive drug), with a continuous MA (meaning a period of use with only a start date), several VVs may be made over time within the scope of this existing agreement. Logistical and emergency instructions may be included in the VV, such as dispense location, request to not include in the GDS (medication distribution system, in Dutch: 'Geneesmiddel Distributie Systeem'), etc.
In the case of a VV, the quantity to be supplied can be stated or the consumption period. With a consumption period, the quantity must be clearly deducible from the dosing instruction of the MA. Note: a consumption period end date has a meaning other than the period of use end date from the MA and may be unequal.
- Period of medication use end date: date until which the pharmacist is allowed to to provide medication (and thereby provide sufficient supplies to the patient for use until that date).
- Period of use end date: date on which the patient must stop the medication (this can be equal to the consumption period end date or further in the future).
Process step: Sending renal function value with prescription
Renal function is important for certain medicines. The renal function value determines the choice of drug and/or drug dosage. It is legally stipulated that if a health professional has performed further research on a patient renal function, he should share abnormal renal function values with the appropriate pharmacist, appointed by the patient (article 6.10, 'regeling geneesmiddelenwet').
The renal function value is always sent with the prescription (using the building block laboratory test results) for medicines for which this is important (characteristic in the G-standard), so that the pharmacist can perform proper medication monitoring. The renal function value should not be older than 13 months, because with stable chronic renal impairment the renal function should be checked at least once a year. 1 month has been added to allow some backlog in practice.
If at the time of sending of an MA and/or VV no renal function is known, then the prescription policy remains unchanged. Sending the laboratory test result renal function value without an MA and/or VV is beyond the scope of this information standard.
Process step: Sending height and weight values with prescription
It is possible for the prescriber to send the body height and weight of the patient with the prescription. This is the height an weigh of the patient that the prescriber used for the medication agreement. Hence, it is possible for these values to diverge (be more accurate) from the height and weight values that are registered in the patient details. This could be necessary for prescribing for children or for medication where weight (e.g. coagulation medication) or height (e.g. oncolytic agents) are important factors to consider.
Body height and weight are separate building blocks. They can only be send together with the prescription and with the medication agreement. The information is not available on request.
Process step: (Actively) making available
This step involves information exchange. Information can be sent or made available with different intentions:
A. As an order for the pharmacist to dispense medication. The prescriber sends the MA to the pharmacist. In an ambulatory situation, the VV is also sent to the patient’s pharmacist. If the pharmacist is not known, this process step may also entail giving out a paper prescription and/or (reactively) making the data available (see C). When the pharmacist has filled the order, the prescriber will receive a notification (see paragraph 2.3.10).
B. As an order for the pharmacist to implement a medication change (including stop-MA with stop type 'permanent' and 'temporary') that impacts or may impact current MVE by this pharmacist. A current MVE indicates an order (as in A) that has been accepted, but has not yet been completely filled. For example, when medication is still being dispensed or when a VV dictates that medication should be dispensed multiple times, but not all have taken place yet, e.g. with the GDS.
C. Making medication data (MA, VV, MGB) and possibly the medication overview available to allow fellow health professionals and/or patients to access these later, possibly in combination with prescription without a specified recipient (see paragraph 9.1).
D. Actively sending (informing) medication data (one or more building blocks) to a specific health professional at the request of the patient when in the presence of this health professional or at discharge.
In case of corrected data, the prescriber assesses who should be actively informed of this correction. This can be done, for example, by sending the new MA (option A or B above) or by means of a telephone consultation. In an ambulatory setting (general practitioner/outpatient clinic) medication data are usually sent or made available directly; in a clinical setting, medication data is usually made available at discharge or interim leave from the institution. This chapter deals with prescription with a specified recipient; paragraph 9.1 details prescription without a specified recipient. See also paragraph 1.3.5 for a further explanation on informing (situation A, B, D) and making available (situation C).
Post-condition
- A new MA may have been created (starting, changing or discontinuing medication)
- A VV may have been made (only outpatient)
- An order may have been sent to the pharmacist to carry out MVE
- An order may have been sent to the pharmacist to modify MVE
- Fellow health professionals and the patient have been informed or may inform themselves about the new medication data: MA, VV, MGB
- Fellow health professionals and the patient have been informed or may inform themselves about a medication overview.
Information systems and transaction groups
The prescriber and the pharmacist as well as other health professionals and users all make use of an information system, respectively, an electronic prescribing system (EVS), a pharmacist information system (AIS, incl. ZAIS), a XIS and a PGO[14]. These information systems each have different system roles which enable the exchange of data between these information systems as part of the prescription process. Chapter 7 includes an overview of all information systems, system roles, transactions, etc. The most important elements for the prescription process are included in the overview below.
Use cases
The following specific use cases have been elobarated:
- Short-term medication
- Continuing medication
- Hard end date for period of medication use
- Medication as needed
- Course of treatment as needed starting in future
- Two dosages of the same medication at the same time
- The same medicinal product with different strengths at the same time
- Explanation in medicatieafspraak with deliberately chosen special characteristic
- New medicatieafspraak, no verstrekkingsverzoek
- New verstrekkingsverzoek under existing medicatieafspraak
- Dosage change (sufficient supply)
- Prescription no longer needed after first verstrekkingsverzoek
- Discontinuing medication
- Temporarily halting/resuming medication
- Temporarily halting for an intervention
- Paper prescription
- Carrying out medication verification and evaluation of foreign or self-care medication
- Day treatment
- Starting with medication before admission
- Emergency admission
- Interim discharge
- Transfer to another institution
- Do not dispense before
- Discontinuation of medication by third parties
- Two PRKs in a single medicamenteuze behandeling
- Creating a medicatieafspraak after the fact
- Single medication use
- Provisional and final medication order
- Inadvertently ‘outstanding’ medication or 'orphans'
- Missing digital medicatieafspraak at admission
- Own articles (90 million numbers)
- Dosing with minimum interval
- Verstrekkingsverzoek with number of repetitions
- Prescribing non-medicines (paragraph 4.1.37)
- Send renal function value in the prescription
- Cancelling a prescription that was sent earlier
- Modification of someone else's medicatieafspraak
- Setting up a variable dosing regimen (WDS)
- Changing a variable dosing regimen (WDS) during period of use
- Stopping medication with a variable dosing regimen (WDS)
Process: dispense
This paragraph describes the process of dispensing medication, including repeat prescriptions and GDS. This process encompasses all actions a pharmacist must take for the patient to not only receive a medicinal product, but to also receive the associated pharmaceutical care ensuring a safe and effective use of the medicinal product by the patient. The dispense process starts with providing pharmaceutical care. If necessary, a medication administration agreement will be made and if needed, medication is dispensed. Medication dispense (i.e. handing out a medicinal product) does not always take place. This may be the case when the medication agreement is changed (e.g., in case of dose reduction where the patient still has enough supply), when the medication is discontinued or, in an ambulatory situation, the medication is not picked up. When the MA and/or the VV do not comply (see paragraph 2.3.5), the prescriber will be informed. Finally, the recorded data are (actively) made available.
Medication assessment is the process in which the physician and pharmacist consider all medication of the patient against the background of his condition, applicable treatment guidelines, the well-being of the patient, etc. Medication assessment is defined in this document as a combination of treatment evaluation (as described in the previous paragraphs) and pharmaceutical care. Depending on the findings, the previously described medication verification and prescription processes are followed, after which medication dispense takes place.
For the GDS, many pharmacists collaborate with another organisation that handles part of the pharmacists’ logistics. This then also requires data exchange with that party. Besides, not all medication can be included in the GDS packaging, which adds to the logistical complexity for the pharmacist. The internal logistics and communication between the pharmacist and his subcontractor(s) are outside the scope of this information standard. It has been established however that with the provision of the current building blocks and the underlying data elements, this logistical process can be adequately supported.
Current situation
The following deviations (from the desired situation that) are currently observed:
- In the current situation, in the case of GDS, the prescribing physician and other health professionals receive a large quantity of dispense messages. This becomes difficult to manage for these health professionals.
- In the current situation, in the case of GDS, pharmacists and general practitioners communicate via so-called authorisation lists. The pharmacist sends an overview of all the patient’s medication to the general practitioner for authorisation. This complicates matters for a general practitioner because he will need to verify all medication agreements. This should not be necessary, as most medication agreements/dispense requests have already been authorised. It would be much more efficient if the general practitioner only needs to authorise those medication agreements/disprense requests that have not been authorised yet, e.g. a new dispense request on the basis of an existing medication agreement.
- In the current situation, a locum pharmacy often does not inform the regular general practitioner and regular pharmacy when medication is dispensed.
- In the current situation, a proposal for a medication agreement may have been aligned with the prescriber over the phone and/or the pharmacist and prescriber have made an agreement about handling a warning from the medication monitoring system.
- The intention of the return message is not always clear: no distinction can be made in the return/delivery message between displaying a physical delivery and a transmission of information about a medication change.
- In the current situation, pharmacists sometimes register (and communicate) medication dispenses when they are preparing the medication for the patient instead of at the time when the medication is actually dispensed to the patient. This means that medication that has not been picked up, is sometimes erroneously registered as dispensed.
Precondition
An MA exists. In an ambulatory situation there may also be a corresponding VV.
Trigger event
The pharmacist starts the medication dispense process on the basis of one of the following events:
- Receipt of an order to make an MVE on the basis of a new MA. In an ambulatory situation, this order is always accompanied by a VV.
- Receipt of an order to process a new MA in an ongoing MVE.
- Receipt of a trigger (for example, via a patient or a repeat module of the pharmacist information system) for a repeat MVE under an existing MVE or a new VVV.
Process step: Providing pharmaceutical care
Pharmaceutical care is provided by a community, outpatient (i.e. at the hospital) or institutional pharmacy, depending on the health professional who has created the MA:
- MA, possibly with a VV from the general practitioner or specialist: care provided by a community or outpatient pharmacy.
- MA from specialists and other prescribers in hospitals/institutions: care provided by an institutional pharmacy or a community pharmacy that supplies the respective institution.
Medication monitoring is also part of pharmaceutical care.
Based on the received MA or a change in the situation of the patient, the pharmacist decides how to apply this by:
- Making one or more new TAs.
- Continuing, permanently discontinuing, temporarily halting or modifying an existing MA.
- Rejecting the MA.
- Proposing a new MA.
- Proposing a new medication dispense (VVV).
The last three situations are explained in the following paragraph. The first two situations are explained in paragraph 2.3.6. In conclusion of provided pharmaceutical care, which may comprise new agreements and medication dispenses, a new up-to-date medication overview may be compiled and made available.
Process step: Informing the prescriber
There is a number of situations in which the pharmacist informs the prescriber, including:
- When a new or modified MA is needed
- When a new VV is needed
For more information about informing, see paragraph 1.3.5.
A new or modified MA is needed:
- When a new TA cannot be created based on the received MA because the pharmacist suspects an error in the MA, or
- After a signal from the medication monitoring system as part of pharmaceutical care. The signal may indicate, for example, that the dosage should be lowered or increased, that it is advisable to select another medicinal product, that a product should be temporarily discontinued, that another additional product should be added, etc., or
- Based on medication use as reported by the patient during pharmaceutical care, or
- When the temporarily halted pharmaceutical treatment may be resumed.
- A TA is not yet created or modified in these situations.
Instead, the pharmacist starts by contacting the prescriber by phone, informing him of the suspected error and proposing an alternative. The pharmacist may also send a digital proposition called VMA to the prescriber. He recommends a specific MA in this proposal, together with the reason and arguments for that recommendation. The prescriber may approve the VMA and make it into a final MA (see also paragraph 2.2.4).
A new VV is needed when the patient’s medication stock is depleted or nearly used and the treatment may need to be continued (request repeat prescription). The patient either requests a repeat MVE from the pharmacist or has signed up in the past for proactive repeat MVE and a notification signal is generated by the repeat module of the AIS when the patient requires new medication[15]. When the existing VV is not adequate, the pharmacist may communicate this request over the phone or send a digital VVV to the prescriber. The VVV may contain indications for the prescriber, such as urgency. The prescriber may approve the received proposal and alter it into a final VV. The prescriber informs the requestor via a AVVV (see also paragraph 2.2.4 and subsequent paragraphs).
Process step: Creating an administration agreement
If the MA and, if applicable, the corresponding VV can be processed, a TA will be created. By creating a TA, the pharmacist fulfills the MA. The TA is communicated to the patient or the person administrating the medication. The TA belongs to the same MBH as the MA it fulfills. As is the case with the corresponding MA, a TA may start in the future. The dosage in the TA may deviate from that in the MA, for example because a certain strength is not in stock. This means that another PRK can become part of the MBH when, for example, 1 tablet of 20 mg is changed into 2 tablets of 10 mg. Before the TA is made (actively) available, medication monitoring will occur in accordance with applicable guidelines, as part of this process step.
On the basis of the TA, an administration list[16] can be compiled for home care or nursing staff, among others.
When creating a TA, the same principle applies as for the MA: each change is recorded in a new TA.
The following paragraphs describe the different situations in which a TA is created: new TA or continuing, permanently discontinuing, temporarily halting or modifying an existing TA.
New administration agreement
In case of a new MA, a new TA is always created. A new TA is also created in case of a new preference policy or a change in stock which results in the selection of a different medicinal product. When creating a new TA, the pharmacist takes into account, among other things:
- Preference policy,
- Inclusion in GDS-packaging,
- Available stock of the institution (‘hospital formulary’) or the pharmacy itself.
If, in an ambulatory situation, the first provision/dispense to the patient occurs later than agreed, the start date of the TA will be different from the start date of the MA.
Continuing an administration agreement
When the existing MA and TA are sufficient to carry out an MVE, the TA will not be adjusted.
Discontinuing an administration agreement
An MA in which it has been agreed to discontinue medication permanently, leads to a stop-TA under the same MBH (this also applies in case of a stop-MA as a result of a change) with stop type ‘permanent’. The stop-TA prevents a new MVE of the discontinued medication. In an ambulatory situation, the prescriber can indicate in the MA that the medication will be discontinued with the next roll (with GDS). In this case, the start date of the stop-TA may be later than indicated in the original stop-TA.
Temporarily interrupting an administration agreement
Temporarily interrupting an MA results in a stop-TA (stop type: 'temporary'). Upon resuming, a new TA is created. Both TAs belong to the same MBH as the MA.
Modifying an administration agreement
A modified MA (leading to a new MA) leads to a new TA under the same MBH. As with the MA, a change to a TA means the termination of the existing TA (a technical stop-TA) and the creation of a new TA.
Process step: Dispense
After creating the TA, the pharmacist prepares the product and dispenses it to:
- The patient in a community setting,
- The patient admitted to a hospital, nursing home or other institution.
The pharmacist records MVE[17].
Medication dispense to patients:
- In an ambulatory situation only occurs on the basis of a VV or a repeat VV,
- In a clinical situation occurs on the basis of the MA without the need for a VV.
Process step: (Actively) making available
This step involves information exchange. Information can be sent or made available with different intentions:
As a request to the prescriber to create a new medication agreement (VMA) or a new dispense request (VVV).
Informing the prescriber about the processing of the prescription (TA and/or MVE).
Making medication data available (VV and MVE) to allow fellow health professionals and/or patients to access these later.
Making a medication overview available to allow fellow health professionals and/or patients to access these later.
Post-condition
- A TA could have been created.
- An MVE could have taken place and, if necessary, the patient has received instructions on how to use the medicinal product.
- Fellow health professionals have been informed or may inform themselves. The prescriber has been informed.
- If necessary, the prescribing physician has been requested to provide a new/modified MA or VV.
Information systems and transaction groups
The prescriber and the pharmacist as well as other health professionals and users all make use of an information system, respectively, an electronic prescribing system (EVS), a pharmacist information system (AIS, incl. ZAIS), a XIS and a PGO[18]. These information systems each have different system roles which enable the exchange of data between these information systems as part of the prescription process. Chapter 7 includes an overview of all information systems, system roles, transactions, etc. The most important elements for the prescription process are included in the overview below.
Use cases
De volgende specifieke use cases van ter hand stellen zijn uitgewerkt:
- New medicatieafspraak, medicatieverstrekking of the same product
- New medicatieafspraak, medicatieverstrekking of the same product
- Existing toedieningsafspraak is adequate
- Medicatieafspraak wanted (informing the prescriber)
- Request and dispense
- Patient requests repeat prescription via physician (reactive repeat)
- Patient requests repeat prescription via pharmacist (informing prescriber)
- Proactive repeat prescription by pharmacist (informing prescriber)
- Dispense based on an existing verstrekkingsverzoek
- Splitting a prescription
- Starting and continuing a GDS
- The pharmacist changes commercial product
- Adding medication to a GDS
- Discontinuing medication in a GDS
- GDS supplier supplies other commercial product
- Parallel administration agreements with GDS- and non-GDS-dispense
- Handling a stop medicatieafspraak
- Medication dispense with someone else’s administration agreement
- Modification of someone else’s administration agreement
- Verstrekkingsverzoek with number of repetitions
Process: administer
This paragraph describes the administration process. This process comprises the compilation of the administration list for (professional) administrators and the administration which is carried out by a (professional) administrator, the patient himself or an informal caregiver. Professional administrators are physicians, nurses and caregivers (home care/institution).
In paragraph 2.4.1, the current situation is described, followed by a description of the new, altered situation.
Current situation
In the current situation, the (professional) administrator, together with the patient, verifies the medication which should be administered, using the available information (paper and/or digital administration list(s), the dosing schedule for, for example, anticoagulant medication, the information on the label), and, subsequently, administers the medication. In case of uncertain administration instructions, the administrator contacts the prescriber or pharmacist. Uncertainties are more likely to occur with an increasing number of prescribers, suppliers and administrators who are involved in the administration process. In the current situation, for administrators, there are several challenges in the transfer of medication data:
- Transfer of medication data between inpatient health care or home care, and hospital health care is missing or is not available in time;
- Medication changes are not received (in time), and medication stops are missing;
- Up-to-date data on the administration list is not available in the case of supply by multiple pharmacies during after-hours care (evening, night or weekend);
- Separate dosing schedules, in addition to the administration list, of (highly) variable dosages, for example anticoagulant medication;
- Problems related to a separate dosing schedule of (highly) variable dosages; for example, loss of the dosing schedule, verbal information regarding changes of the dosing schedule;
- Medication as needed, injection schedules (for example, for insulin) are missing on the administration list (the injection schedules will be worked out in the information standard in the future);
- An overview is missing of all health professionals and healthcare providers which are involved.
In the current situation, the pharmacist compiles the administration list for the (professional) administrator or the patient. This administration list comprises the administration times, which are geared to the rounds of the home care organization, with account for the pharmaceutical ranges. A paper or digital administration list is used by the (professional) administrators for the registration of the medication administration. A paper administration list (including the administration registration) is in the patient’s home, and is therefore accessible for all professional administrators (irrespective of organization). The digitally recorded medication administrations are only exchanged with other health professionals in exceptional situations (for example, it may be exchanged in the case of admission or special circumstances). With the transition to an electronic administration registration, the administration list (with the recorded administration times) is no longer physically present; instead the administration is recorded in the E-TDR/E-TRS (electronic administration registration/electronic administration registration system) (see paragraph 2.4.8). The involved professional administrators of different organizations (with possibly different E-TRS applications) cannot consult or can only consult with difficulty the registered administration procedures of other organizations, because of registration in different applications and/or databases.
Precondition
The patient administers medication to himself or the patient must be administered medication by a(n) (professional) administrator and, therefore, requires an administration list.
Trigger event
The moment the medicinal products must be administered.
Process step: Administration list
The (professional) administrator or the patient receives or asks for the (actively) made available medication data that is required for automatic generation of the administration list. For the administration list, the building blocks ‘medicatieafspraak’ (MA), ‘wisselend doseerschema’ (WDS), ‘toedieningsafspraak’(TA), ‘medicatieverstrekking’ (MVE) and ‘medicatietoediening’ (MTD) are needed. In order to compile a complete administration list, the (guide) administration times and dosing instructions are required. The pharmacist and prescriber can enter these data in the TA, and MA or WDS, respectively. In general, pharmacists and prescribers in the ambulatory setting do not register the (guide) administration times and, therefore, they should receive a trigger which indicates that it concerns an ‘administration patient’; a patient who is aided in the medication administration or a patient who requires an administration list. For some medication, it is necessary to provide information on the previous ‘prick and patch locations’ (sites of administration); this is registered in the MTD. In the case of inpatient health care, administration lists are available for all patients, and (guide) administration times are registered by default.
Most (guide) administration times are chosen based on the administration times of other prescribed medication and the rounds of the administrator, especially for MAs and TAs with standard flexible (guide) administration times. Further agreements should be made regarding the (guide) administration times by those concerned in the healthcare field (for example, which deviations from the (guide) administration times are permitted). If the administration of a drug requires a specific time of administration, for example because of medication interactions, this is communicated by entering in the TA and/or MA that the (guide) administration time is not flexible.
The administration list differentiates between GDS medication (GDS: medication distribution system, in Dutch ‘Geneesmiddel Distributie Systeem’) and non-GDS medication based on the distribution type. This information is recorded in the building block MVE. Subsequently, the administrator (administration software) compiles the administration list, based on the MA, WDS, TA and MTD, with differentiation according to administration moment. Prescribers, pharmacists and administrators (PrickPatchLocation) are responsible for providing the medication data which is necessary for generating the administration list.
NB. It is currently explored in which way information regarding GDS can be best exchanged.
Process step: Administering
The (professional) administrator has received the medication data (MA, WDS, TA, MVE and MTD) that is required for the medication administration. These data is presented in the administration list as described above. The (professional) administrator, together with the patient, verifies the available medication and the administration data. If agreed and needed, the professional administrator prepares the medication for administration. The medication is administered and the administrator records the administration in the information system or on the administration list. Deviations in the medication administration (not administered, adjusted dosage, refusal by the patient, swallowing problems, adverse effects, etc.) are recorded as well.
Process step: (Actively) making available
In this step, information is exchanged. The administration data can be sent or be made available with the following aims:
- The recorded MTDs and deviations can be sent for information to a fellow health professional;
- The MTD can be made available so that the data can be accessed later by fellow health professionals and/or the patient. For example, the administration data can be retrieved upon transfer of the patient to another department or institution. Also, a health professional can check which medication is administered to a patient.
Post-condition
The patient has administered medication by himself or has been administered medication. The MTDs can be recorded in an information system and can be (actively) made available for fellow health professionals and/or the patient.
Information systems and transaction groups
The prescriber and the pharmacist as well as other administrators and patients (optional) all make use of an information system, respectively, an electronic prescribing system (‘elektronisch voorschrijfsysteem’ - EVS), an electronic client file (‘elektronisch cliëntendossier’ - ECD), a pharmacist information system (‘apothekersinformatiesysteem’ - AIS), a hospital pharmacist information system (‘ziekenhuisapotheekinformatiesysteem’ - ZAIS), an administration registration system (‘toedieningsregistratiesysteem’ - TRS), a thrombosis service information system (‘trombosedienstinformatiesysteem’ - TrIS) and a personal health environment (‘persoonlijke gezondheidsomgeving’ - PGO). These information systems each have different system roles which enable the exchange of data between these information systems as part of the administration process. The information systems may have a function in compiling an administration list, and in the registration, the exchange and the delivering of an MTD. Chapter 6 provides an example of compiling an administration list. The most important elements for the administration process are included in the overview below.
Use cases
The following use cases for medication administration have been worked out:
- Creating an administration list
- Exact administration times required
- Missing (guide) administration times
- Non-GDS medication as needed
- Medication supply by multiple pharmacies
- Change in GDS from the next supply or immediately
- Increasing dosage of GDS in new MBH
- Decreasing dosage of GDS in new MBH
- Change processed by the pharmacist
- Change not processed by the pharmacist
- Variable-dosing regimen
- Additional information
- Medication administration deviates from administration list
- Medication administration without medication agreement and administration agreement
- Medication administration of self-care medication
- Correction/cancellation of an administration
- Medication administration on hold
- Medication administration by a prescriber
- Multiple administration organizations
Process: medication use
This paragraph describes the process of medication use including registration of medication use by the patient or a health professional. The information recorded by the patient or health professional may be used, among other things, for medication verification by the health professional. During medication verification, medication use is established by the health professional.
Current situation
- There is currently a limited number of information systems available in which the patient can record his own use of medication. Exchange to health professionals is also highly dependent on the information system used and is often limited to one specific health professional via, for example, one specific platform/app.
- The current medication profiles are often incomplete and not up to date.
Precondition
The patient has been prescribed medication.
Trigger event
The patient has used the medication or does not use it (anymore).
Process step: Medication use
The patient uses the prescribed medication, selfcare medication. Medication use may be recorded by
- The patient himself or his informal caregiver,
- A home care or institution nurse and/or
- Another health professional.
This third option often applies in the event of medication verification (see paragraph 2.1). A variety of information systems is available to record medication use: PHR, XIS, EPF [electronic patient file] / ECF [electronic client file], app, etc.
The following can be recorded as medication use:
- Self-care and other proprietary medication,
- Discrepancies compared to agreements made,
- Confirmation of medication use (promoting compliance),
- Verified medication (see medication verification),
- Changes as a result of, for example, side effects (by the patient himself; a health professional will record this in a different way).
Patients who are being administered medication by a nurse sometimes take the medication by themselves at a later time, depending on the BEM-score (assessment of medication self-management, in Dutch: 'Beoordeling Eigen Beheer Medicatie'); in that case, MTD data may deviate from MGB.
While medication is being used, pharmaceutical care is provided by the pharmacist (see paragraph 2.3.4, e.g. in case of new laboratory values). There may also be follow-up contact during which the pharmaceutical treatment is evaluated (see paragraph 2.2.4).
Recording of medication use by the patient
The patients makes use of a medication profile and indicates actual medication use for each medicinal product for which this is relevant. The patient can indicate whether or not he is using the product and/or whether this is ‘medication use according to agreement’ (whereby the MA and/or TA is displayed) or if there were deviations. In case of deviations, it is possible to enter specific deviations (i.e. with regard to the schedule actually followed by the patient), but deviations can also be entered in more general terms (for example, I take the medication: ‘from time to time’, [x] times per [days/week] on average’, ‘1x daily instead of 2x’, ‘not anymore since 22 January 2015’ or ‘not anymore since about a month’). In case of deviation from the agreements, the patient also indicates the reason for changing or discontinuing.
The patient may also add his own medication to the overview and indicate any side effects as the reason for the changes.
Information about medication use will not always be present. There is also no certainty about the reliability of the information. Sometimes there are agreements between the health professional and the patient about keeping track of medication use, and sometimes the initiative is the patient’s entirely.
Recording of medication use by health professionals
During the process of medication verification (paragraph 2.1) or evaluating a pharmaceutical treatment (2.2.4) the patient (or his informal caregiver) indicates, for example, that he does not use medication or uses it differently than was agreed. He may also indicate that he uses other medication as well (self-care products or foreign medication). The health professional can record these data as MGB. The data about medication use is recorded in addition to the primary medication data with the patient being recorded as the source of the information and the health professional as the author.
Instead of or in addition to recording the MGB, a prescriber may also choose to make a new or modified MA with the patient (in accordance with the process described in paragraph 2.2.5). This may occur when the prescriber sees reasons to adjust the agreement in response to the patient’s actual medication use. When the prescriber sees no reasons to adjust the agreement, he may choose to only record the medication use, perhaps with the remark that he has requested the patient to comply with the existing agreements.
In case of deviations in MGB, a pharmacist may create a VMA for the prescriber (see paragraph 2.3.5) and/or advise the patient to inform the prescriber about the deviations.
The assessment of medication use (effect and side effect) will be recorded by the physician in the medical history or used as a reason to modify or discontinue a medication agreement.
Process step: (Actively) making available
The recorded medication data (MGB) and possibly the medication overview may be sent for information to a fellow health professionals or be made available so that the data can be accessed later.
Process step: Informing the prescriber
The patient may also directly inform the prescriber when a new or modified MA or new VV is needed. The process is similar to the process of the pharmacist informing the prescriber (paragraph 2.3.5).
Post-condition
The medication list has been updated by the patient and actual medication use has been added. If necessary, the patient has asked the prescriber for a new or modified MA or VV.
Information systems and transaction groups
The prescriber and the pharmacist as well as other health professionals and users all make use of an information system, respectively, an electronic prescribing system (EVS), a pharmacist information system (AIS, incl. ZAIS), a XIS and a PGO[19]. These information systems each have different system roles which enable the exchange of data between these information systems as part of the prescription process.
Chapter 7 includes an overview of all information systems, system roles, transactions, etc. The most important elements for the prescription process are included in the overview below.
Use cases
The use cases for medication use are described on the basis of registration by the patient. The prescriber may record medication use in the same way but will rather record the assessment of medication use (effect and side effect) in the medical history or use it as a reason to modify or discontinue a medication agreement.
The following specific use cases for medication use have been worked out:
- Self-care product
- Medication from abroad
- Modification at the patient’s initiative
- Discontinuation of medication at the patient’s initiative
- No more supply
- Feedback to patient through a medication adherence app
- Registrations of abnormal medicatiegebruik by patient due to adverse drug reactions
- Register medicatiegebruik based on provision
Domain-specific handling of the medication process
After Hours General Practice clinics (HAP)
The after hours general practitioner (AHGP) works on behalf of the regular general practitioner (GP). The AHGP may (among other things) start, modify and discontinue MAs in accordance with the process described in paragraph 2.1. If necessary, the AHGP will also create the corresponding VVs.
The AHGP will inform the regular GP about the observation. Currently, it has been agreed that the AHGP does not act as a source of information for other health professionals of this patient. This means that the AHGP will not carry out the process step ‘(actively) making available’. Instead, the regular GP will make the relevant information available to the fellow health professionals. An exception may be made for a prescription created by the AHGP without a specified recipient, see paragraph 9.1.
In principle, the AHGP will follow the generic prescription process, except that a AHGP will carry out certain steps on behalf of the regular GP (‘delegated’).
Mental health care
There are only few planned admissions in mental health care (almost only in departments for eating disorders, clinical rehabilitation, etc.). Most admissions are admissions as a result of acute crisis situations and are therefore comparable to admissions via the emergency departments in hospitals (see also paragraph 4.1.20).
Current situation
In the current situation, patient or family/informal caregivers are asked which medication the patient is using. In most cases, the patient is unable to give an answer (often the reason for admission). Family/informal caregivers (if known) are also not always able to answer this. If this is the case, the admitting physician/psychiatrists will contact the general practitioner or the pharmacist to find out the medication. This is difficult outside office hours and during weekends. When data do come in, only part of them is retyped in the information system of the health professionals.
Process
In the new situation, it will be possible to retrieve the available medication data and medication overviews. On this basis, medication verification, assessment of the medicamenteuze behandeling (as soon as possible) and medication administration (in accordance with Chapter 2) can be started. Medication dispense is carried out by public pharmacists on an outpatient basis and, in clinical practice, often by a contracted hospital pharmacy.
As is the case with hospital discharge, medicamenteuze behandeling will be assessed at discharge from a mental health institution (paragraph 2.2.4 and subsequent). Clinical medication is discontinued and, if necessary, new medication is prescribed or temporarily halted outpatient medication is resumed or permanently discontinued. The physician/psychiatrist focuses mainly on psychiatric medication; somatic medication usually remains unaffected.
Letting the psychiatric patient record his own medicatiegebruik may be experienced as undesirable control instead of it stimulating proper medication use.
The medication data will be made available.
Nursing, care and home care (VVT)
The medication process in a VVT institution (nursing home, care home and home care) is similar to that of the clinical situation. However, in a VVT institution, a geriatrics specialist is responsible for prescribing medication to admitted clients and medication is being dispensed by one or more community pharmacists, possibly as part of GDS if needed. VVT institutions and home care make use of administration lists, which are drawn up based on the toedieningafspraken. The administration list is used to check medication before administering it and to sign off actual medicatiegebruik. Communication of the administration list between pharmacist, prescriber and nursing staff will be explained in more details further on in this information standard.
Hospital admission and discharge
A special situation in the transfer of medication data occurs when a patient is admitted to a hospital. At the moment of admission and of discharge, a lot of changes can take place in the medicatieafspraken and toedieningsafspraken. That is why this subject is separately illustrated in this chapter. This process is described in more detail in instruction manuals for caregivers and software suppliers.
Prior to admission
Before a patient is admitted to a hospital, changes in medication may be necessary. The medical specialist and patient can agree upon starting new medication or stopping continuous medication a few days before admission. The medical specialist subsequently registers a medicatieafspraak or stop-MA and mentions in the explanatory notes how many days before admission the patient has to start or stop the medication. When this medicatieafspraak is registered, the date of admission is often yet unknown. That is why the medical specialist records that this medicatieafspraak depends upon a hospital admission. This means that the start- or end date are uncertain. Technically, this is solved by registering the explanatory notes in the element ‘Condition’ of the period of use of the medicatieafspraak. If this condition is entered, it is clear to caregivers that the period of use is uncertain.
During admission and at time of discharge
When a patient is admitted, the following situations can occur, concerning the outpatient medication:
- Unchanged continuous use,
- Change in dosage,
- Generic substitution (active ingredient remains the same),
- Pharmacological substitution (active ingredient changes, but medication is registered for the same indication),
- (Temporary) stop.
Below, for each situation, an explanation is given for registration of the medication of the patient.
Unchanged continuous use
The patient continues using the outpatient medication during admission.
Both medicatieafspraak and toedieningsafspraak continue during admission and after discharge.
Change in dosage
The patient uses the outpatient medication during admission, but in a different dosage.
The medicatieafspraak for outpatient medication is stopped at admission. During admission, a new medicatieafspraak is registered with the changed dosage. At discharge, the prescriber decides which dosage will apply for the outpatient medication. Depending on this decision, the medicatieafspraak that applied before admission will be continued, or a new medicatieafspraak will be registered. The toedieningsafspraak is also stopped at admission. During admission, a new toedieningsafspraak is registered, based on the new medicatieafspraak. At discharge, the toedieningsafspraak that applied before admission will be continued, or a new toedieningsafspraak will be registered.
Generic substitution
The patient uses a different medicinal product during admission, but with the same active ingredient and dosage as in the outpatient medication.
The medicatieafspraak for outpatient medication continues during admission and after discharge. The toedieningsafspraak for outpatient medication is stopped at admission and continued at discharge. During admission, a new toedieningsafspraak is registered. This toedieningsafspraak will be stopped at discharge.
Pharmacological substitution
The patient uses a different medicinal product during admission, with a different active ingredient from the outpatient medication. This medicinal product, however, is registered for the same indication as the outpatient medication.
The medicatieafspraak for outpatient medication is stopped at admission and will be continued again at discharge. During admission, a new medicatieafspraak is registered. This medicatieafspraak will be stopped at discharge. The toedieningsafspraak for outpatient medication is also stopped at admission and continued at discharge. During admission, a new toedieningsafspraak is registered. This toedieningsafspraak will be stopped at discharge.
(Temporary) stop
The patient (temporarily) stops using the outpatient medication during admission.
The medicatieafspraak for outpatient medication is (temporarily) stopped at admission. If the medication is stopped temporarily, this medicatieafspraak will be continued at discharge. If the medication is stopped definitively, the medicatieafspraak will not be continued. The toedieningsafspraak for outpatient medication is also (temporarily) stopped at admission. If the medication is stopped temporarily, this toedieningsafspraak will be continued at discharge. If the medication is stopped definitively, the toedieningsafspraak will not be continued.
Description of use cases
This chapter contains a description of various use cases. Specific practical situations have been described for the various subprocesses. A large number of the practical situations are based on general medical practice, but are illustrative of similar situations in a different setting. This chapter assumes the knowledge as previously described in Chapter 2.
Use cases, Prescribe
Short-term medication
A 35 year old female patient with a history of urinary tract infection brings her urine to the doctor’s assistant and tells her she has the same symptoms as the previous times. The assistant enquires about other symptoms such as fever and pain in the flank and checks the urine. There are no other symptoms and the urine reveals a urinary tract infection. She tells the patient that the general practitioner will prescribe a course of treatment and that she can pick it up from the pharmacist later. The General practitioner checks the previous courses of treatment and any cultures and records a medicatieafspraak. On 27 January it was agreed:
- Nitrofurantoin CR capsule, 100 mg, 2x daily, 1 capsule, from now on[20] for 5 days.
He records this medicatieafspraak in his information system. The general practitioner then immediately also makes a verstrekkingsverzoek for the pharmacist chosen by the patient:
- Nitrofurantoin CR capsule, 100 mg; 10 capsules.
The general practitioner also records this verstrekkingsverzoek in his information system.
The general practitioner consults with the patient to find out from which pharmacist she wants to pick up the medication. The general practitioner can then inform this pharmacist about the verstrekkingsverzoek and the medicatieafspraak.
The information system of the general practitioner makes this information (verstrekkingsverzoek and medicatieafspraak) available to the other health professionals of this patient.
Continuing medication
The process for continuing medication is practically the same as for short-term medication (see paragraph 4.1.1), except that in the case of continuing medication the medicatieafspraak is made for an indefinite period of time.
For example: the prescriber agrees with a patient who has hypertension that he should use a diuretic on a continuous basis. On 30 March 2013 it was agreed:
- Hydrochlorothiazide tablet, 12.5 mg; 1x daily, 1 tablet; from now on for an indefinite period.
For example, the prescriber chooses in the verstrekkingsverzoek for an initial dispensing quantity of 100 pieces.
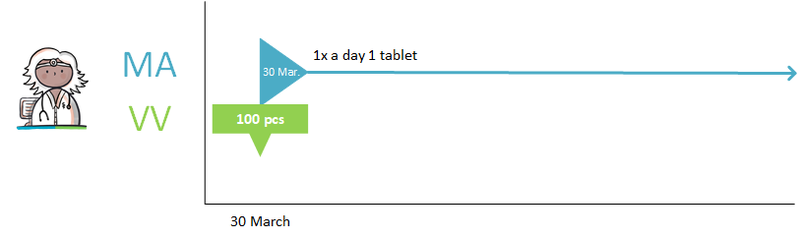
Hard end date for period of medication use
A start date, end date and/or duration can be included in the period of medication use of a medicatieafspraak. If a hard end date is desired, this must also be explicitly stated in the explanation. Including an end date alone is not sufficient because this does not provide enough clarity about the intention of the prescriber.
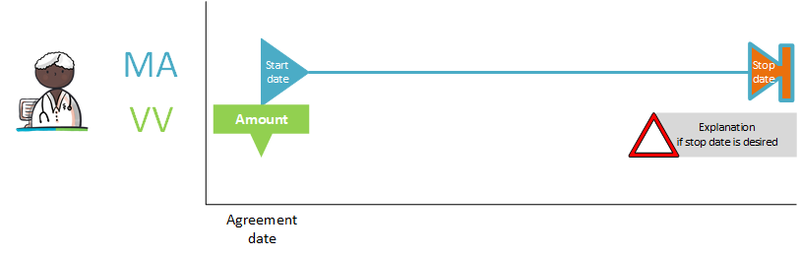
Medication as needed
A 31-year-old female patient has had headaches a few times in a year, with migraine now being diagnosed. The general practitioner prescribes:
- Rizatriptan tablets, 10 mg, as needed 1c1t under the tongue. First tablet at the next clear migraine attack.
De huisarts legt de medicatieafspraak vast en doet een verstrekkingsverzoek:
- 6 sublingual rizatriptan tablets, 10 mg.
Course of treatment as needed starting in future
A 60-year-old patient with post-thrombotic syndrome experiences erysipelas (severe infection of the leg which can require admission if medication is not started quickly) sometimes every other year, sometimes three times a year. At the request of the patient, the general practitioner prescribes an antibiotic treatment the patient can start immediately in case of a recurrence of erysipelas. The following medicatieafspraak is created:
- Flucloxacillin capsules, 500 mg, 1 capsule 4x daily, for 10 days. In the medicatieafspraak is indicated (if necessary): start in case of recurrence, as needed.
The general practitioner immediately creates a verstrekkingsverzoek:
- Flucloxacilline 500 mg capsules 40 stuks.
Two dosages of the same medication at the same time
A 79-year-old man with metastatic prostate cancer is increasingly suffering from pain and his medication is being changed. The specialist prescribes oxycodone tablets, 10 mg, 1 tablet 4x daily and as needed for pain during the night, 1 tablet 1x daily, on October 9. The specialist records this in one single medicatieafspraak with two dosing instructions and creates a verstrekkingsverzoek for 60 tablets of oxycodone of 10 mg.
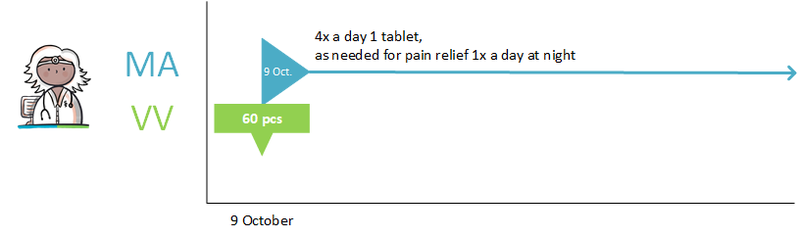
The same medicinal product with different strengths at the same time
The same 79-year-old patient (see paragraph 4.1.6) is experiencing even more pain. The specialist discontinues the 10 mg tablets of oxycodone on October 16 and prescribes 20 mg retard tablets of oxycodone, 2 tablets 3x daily, and 20 mg normal release tablets of oxycodone as needed in case of nocturnal pain, 1 tablet 1x daily. The specialist records this in two medicatieafspraken (with, for the time being, two separate medicamenteuze behandelings) and creates a verstrekkingsverzoek for both medicatieafspraken, respectively 45 retard tablets of 20 mg oxycodone and 30 tablets of 20 mg oxycodone (normal release).
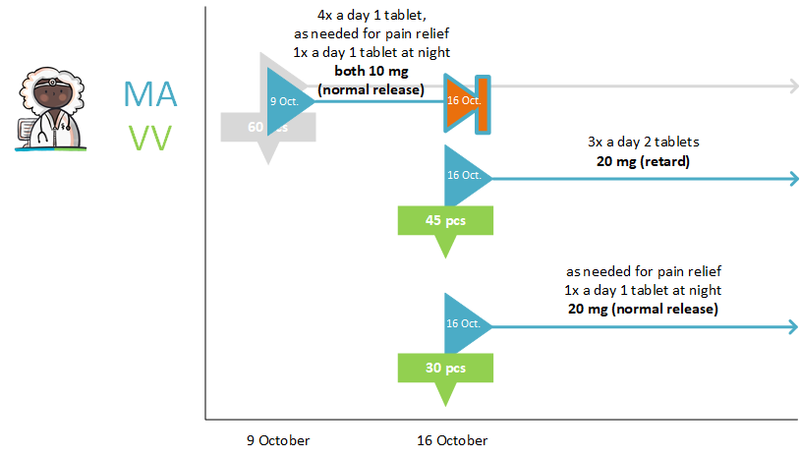
Explanation in medicatieafspraak with deliberately chosen special characteristic
This use case is described in the zib medicatieafspraak/additional information. Here it is described for information purposes only. When selecting a medicinal product, it is possible to deviate from what is expected or standard. For example, when a hospital uses a different formulary than a community pharmacy. For reasons of efficiency the hospital has opted for a single gastric acid inhibitor: pantoprazole.
At admission, a patient with omeprazole is switched to pantoprazole for the duration of the hospitalization. At discharge, the patient returns to omeprazole.
It is clear that this may sometimes go wrong and that the patient might end up taking both omeprazole and pantoprazole if nothing is done. In the medicatieafspraak of the hospital for pantoprazole, a remark can be made about this deviation in order to make it clear that pantoprazole is the replacement of omeprazole or that it has to be used in addition to omeprazole.
Half-strengths are another example. The hospital sometimes as tablets available with half the strength of the normal commercial preparation (proprietary production). When a patient enters the hospital with a prescription of 25 mg of chlorthalidone, half a tablet 1x daily, he will receive 12.5 mg of chlorthalidone, 1 tablet 1x daily. In this specific case, this means that nursing staff does not have to break tablets in half. There is a risk that the patient will use the 25 mg again at home, but now a whole tablet at the time. An explanation in the medicatieafspraak (additional information) regarding the last 25 mg of chlorthalidone, makes it possible to indicate whether this was a deliberate increase.
If a prescribed product has to be a very specific commercial product, this can be indicated with ‘medical necessity’.
New medicatieafspraak, no verstrekkingsverzoek
A 50-year-old man comes to the general practitioner with back problems. The symptoms have been present for three weeks and he is already using paracetamol. The general practitioner agrees with the patient that he can additionally use diclofenac:
On 30 January it was agreed:
- Diclofenac tablet, 50 mg, 1 tablet 3x daily, from now on for 3 weeks.
He records this medicatieafspraak in his information system. The patient indicates there is sufficient supply at home: his wife still has an ample supply of diclofenac left because of a different problem a year ago.
A verstrekkingsverzoek is therefore not needed and the pharmacist also does not dispense any medication.
The general practitioner makes the new medicatieafspraak available to any fellow health professionals of this patient. This allows the regular pharmacist to inform himself about this new medicatieafspraak.
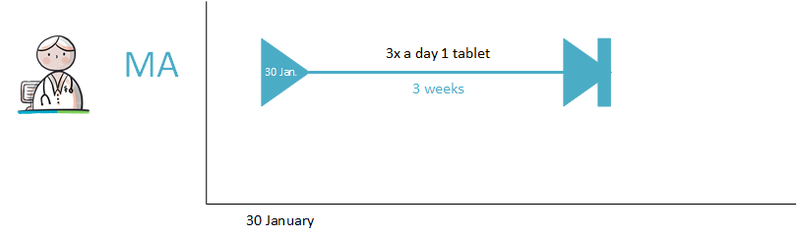
Another example: Mr Simons receives medication from the pharmacy weekly. In the past there were many requests for extra medication, which led to clear agreements about the dispense policy.
This is about:
- Medicatieafspraak: Diazepam, 5 mg, 1 tablet 4x daily, from 1 January 2012 for an indefinite period
- Verstrekkingsverzoek: 28 tablets with 10 repeats; remark: weekly medicatieverstrekking
The verstrekkingsverzoek is repeated approximately every 11 weeks. The last verstrekkingsverzoek was on 3 March 2016:
- one week (28 tablets) with 10 repeats
This way, the pharmacy can go on for 11 weeks.
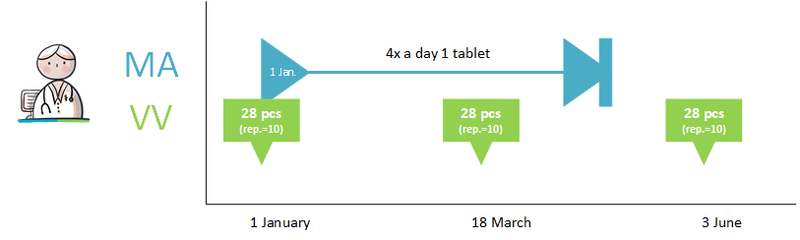
The last few years have been quiet. Mr. Simons is no longer asking for extra medication. At the last consultation he indicated that diazepam, 3x daily, is sufficient. This has been recorded in a medicatieafspraak:
- 1 April 2016 Diazepam, 5 mg, 1 tablet 3x daily, from 1 April 2016 for an indefinite period.
The previous medicatieafspraak will be terminated as of 31 March 2016 (see discontinuation of medication in paragraph 2.2.5).
In the current situation, the general practitioner would have called the pharmacy to make sure that 21 tablets of diazepam would be given with the following medicatieverstrekking, instead of 28. No new verstrekkingsverzoek would have been needed at the time.
In the new situation, the general practitioner sends the new medicatieafspraak to the pharmacy. Based on the new medicatieafspraak, the pharmacy provides 21 tablets per week, the previous verstrekkingsverzoek is still sufficient.
New verstrekkingsverzoek under existing medicatieafspraak
A prescriber may also create a new verstrekkingsverzoek as part of an existing medicatieafspraak. This medicatieafspraak may have been created by a different prescriber, for example a psychiatrist. This concerns repeat medication. This use case is described in paragraph 4.2.6.
Dosage change (sufficient supply)
Patient (37 years old, asthma) visited the pulmonologist on 13 August 2013 for a check-up of his asthma. The pulmonologist has established that patient’s asthma is not adequately controlled.
The patient is currently using beclomethasone in accordance with a previously made and registered medicatieafspraak: on 10 June 2010 it was agreed:
- Beclomethasone aerosol, 100 microgram/dose, inhaler; 1 inhalation 2x daily; from 10 June 2010 for an indefinite period.
On 13 August 2013, the pulmonologist agrees with the patient on a dosage increase:
- Beclomethasone aerosol, 100 microgram/dose, inhaler; 2 inhalations 2x daily; from 13 August 2013 for an indefinite period; reason for adjustment: insufficient effect.
The previous medicatieafspraak of 10 June 2010 it is no longer valid: it is terminated (see Changing medication in paragraph 2.2.5). The patient still has a sufficient supply, a verstrekkingsverzoek is therefore not needed.
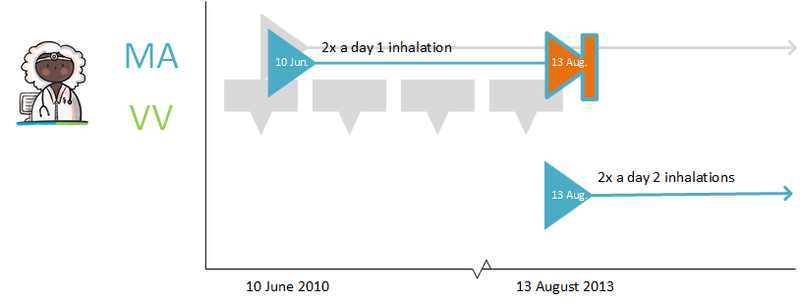
In case the patient has no more supply, a new verstrekkingsverzoek will be created.
The information system of the pulmonologist makes the new medicatieafspraak available to the other health professionals of this patient. This modification is received by the pharmacist (fellow health professional). The pharmacist processes the modification. The pharmacist may also choose to accept the modifications automatically.
Prescription no longer needed after first verstrekkingsverzoek
A 16-year-old woman has a boyfriend and does not want to become pregnant. After explanation she opts for the pill. The general practitioner prescribes ethinylestradiol/levonorgestrel tablets of 20/100 µg, 1 tablet 1x daily for 21 days, then no tablet for 7 days, then another 21 days of taking a tablet once a day. Start on the first day of the next menstruation. The general practitioner records the medicatieafspraak and creates a verstrekkingsverzoek for 63 coated microgynon 20 tablets. He explains to her that if she does not experience any problems, she can continue to get the pill via the pharmacy.
Three months later, the woman sends a request for repeat supply to the pharmacy. The pharmacy provides the product and communicates the medicatieverstrekking to the general practitioner.
Discontinuing medication
Patient (37 years old, asthma) visits the pulmonologist on 13 August 2013 for a check-up of his asthma. The patient is suffering from a side effect. The patient is currently using Beclomethasone in accordance with a previously made and registered medicatieafspraak. On 10 June 2010 it was agreed:
- Beclometasone aerosol, 100 microgram/dose, inhaler; 1 inhalation 2x daily; from 10 June 2010 for an indefinite period.
On 13 August 2013, the pulmonologist agrees with the patient to discontinue the medication (medicamenteuze behandeling is discontinued). Medication verification is also applicable here. In addition, medication monitoring may also be relevant here, for example if gastroprotrective drugs are being used because of the use of the medication to be discontinued (the information system will not always signal this automatically).
The pulmonologist records:
- Beclometasone aerosol, 100 microgram/dose, inhaler; stoptype ‘definite’; from 13 August 2013. Because of a side effect.
This modification is received by the pharmacist (fellow health professional). The pharmacist processes the modification. The pharmacist may also choose to accept the modifications automatically. The pulmonologist decides to also actively inform a fellow health professional (such as a specialist) about this (part of the process step (actively) making available).
The information system of the pulmonologist makes this information (agreement to discontinue) available to the other health professionals of this patient.
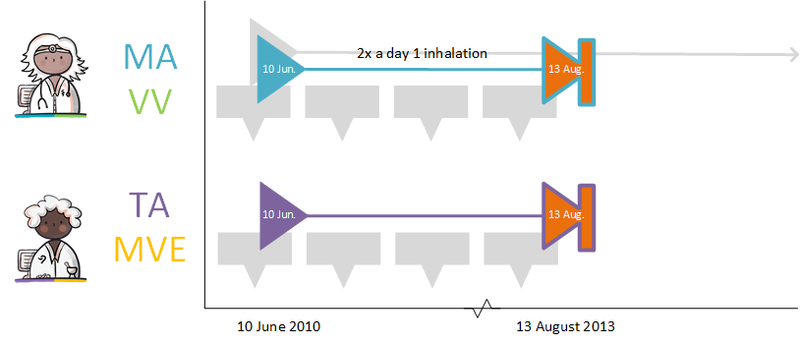
Temporarily halting/resuming medication
A patient is being treated with simvastatin (cholesterol-lowering agent): 40 mg, 1 tablet 1x daily, for an indefinite period. Because of a throat infection in combination with an allergy for the first antibiotic of choice, Clarithromycin, 250 mg, 1 tablet 2x daily (antibiotic) is prescribed for 1 week. During that week, simvastatin is temporarily halted because of an interaction.
The general practitioner records:
- Simvastatin 40 mg; halt temporarily; for 1 week; reason: interaction
- Clarithromycin, 250 mg; 1 tablet 2x daily; for 1 week (and a corresponding verstrekkingsverzoek)
The pharmacist is actively informed by the general practitioner about the temporary halt of simvastatin. A new medicatieafspraak is also created with which simvastatin is resumed after one week. The pharmacist will also receive the medicatieafspraak and the corresponding verstrekkingsverzoek for clarithromycin. See also Temporarily halting and resuming medication in paragraph 2.2.5.
Temporarily halting for an intervention
A 62-year-old man uses acetylsalicylic acid, 100 mg, 1 tablet 1x daily because of coronary disease. He has an intestinal polyp that must be removed. The general practitioner informs him that he should stop using acetylsalicylic acid three days before the intervention and resume the day after the intervention. The general practitioner records the temporary halt in a medicatieafspraak with an enddate 3 days before the intervention; stoptype ‘temporary’; reason for halting: ‘intervention’. In the (second) medicatieafspraak for presuming, 1 day after the intervention is used as the start date. The general practitioner actively informs the pharmacist and the gastroenterologist about the medicatieafspraken.
When the date of the intervention is unknown or uncertain, the explanation will indicate that the medication should be halted three days before the intervention and resumed one day later. The duration of the temporary halt is then 4 days. See also Temporarily halting and resuming medication in paragraph 2.2.5 and Prior to admission in paragraaf 3.4.1.
Paper prescription
The physician gives the patient a paper prescription[21]. A paper prescription contains a medicatieafspraak and a verstrekkingsverzoek. The patient can use this prescription to pick up the medication from any pharmacist. The general practitioner does not inform the issuing pharmacist in this case. However, the process is actually the same: the activity ‘(actively) making available’ is now only ‘handled’ by the paper prescription in the hands of the patient. The supplying pharmacist will copy this paper medicatieafspraak and verstrekkingsverzoek in his own information system and complete it with a toedieningsafspraak. The pharmacist may also retrieve the medicatieafspraak and verstrekkingsverzoek that were made available digitally and process them in his own information system this way.
The information system of the general practitioner makes the new medicatieafspraak and verstrekkingsverzoek available for fellow health professionals. When the information system of the general practitioner has not made these available this may be because it concerns a toedieningsafpsraak without digitally available medicatieafspraak and verstrekkingsverzoek. The pharmacist will not make the medicatieafspraak and verstrekkingsverzoek obtained ‘from paper’ available because he is not the owner. Next to that, this could lead to, possibly only in the future, wrongful duplication of medicatieafspraken and verstrekkingsverzoeken.
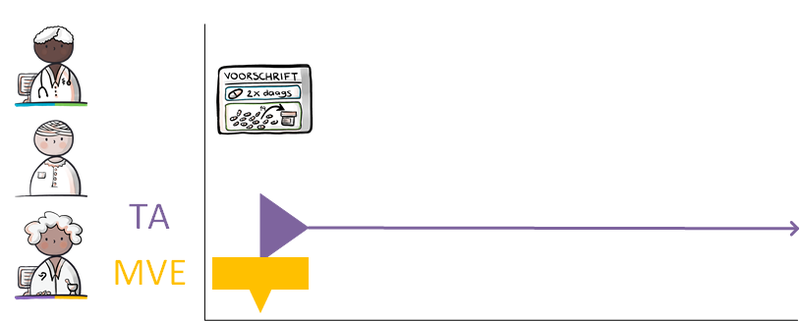
Carrying out medication verification and evaluation of foreign or self-care medication
When medication cannot be found in the information system (such as foreign medication and self-care medication), this medication will be recorded as medicatiegebruik (see paragraph 2.2.5).
Day treatment
An admission for day treatment is comparable with an outpatient consultation or an emergency admission during which the medication is supplied by the hospital pharmacist. In the case of admission for a day, extensive medication verification does not usually occur. In the case of admission for a day, the medication prescribed is recorded (often on the basis of protocols according to which verification is carried out afterwards) and administered.
For treatments with cytostatics, for example, there is a medicatieafspraak, but it is not always clear in advance when the medicatieverstrekking/administration takes place. Reason: treatments are often postponed and then supplied and administered later.
Starting with medication before admission
Prior to cataract surgery, Nevanac is started three days before the surgery. The eye drops are used until three weeks after surgery (a total duration of medication use of 24 days). The specialist creates a medicatieafspraak for Nevanac, 1 drop in the morning, for 24 days. When the date of the surgery is unknown or uncertain, the explanation may indicate that Nevanac should be started 3 days before surgery takes place. See Prior to admission in paragraaf 3.4.1
Emergency admission
In the event of an emergency admission, extensive medication verification is not carried out beforehand as would normally be the case with clinical admissions. Agreeing on medication with the patient is also often not possible in the event of an emergency admission. Often, the patient is administered medication as soon as possible and verification only takes place afterward
Interim discharge
When a patient temporarily leaves the hospital/institution to go home, for example, for weekend leave, the clinical medication continues. In order to keep the patient’s own general practitioner informed, the medication overview is provided to the patient and the medication data are made available.
Transfer to another institution
In case of a transfer to another institution, the medicamenteuze behandeling is evaluated (see Chapter 2.2.4 and paragraph 2.2.5). Medication overview and medication data are (actively) made available. The new attending physician evaluates the medicamenteuze behandeling and determines the institutional medication.
Do not dispense before
The prescribing physician creates a verstrekkingsverzoek on 14 April 2017, but wants to indicate in that same verstrekkingsverzoek the medicatieverstrekking may only take place on or from a later date, for example, 23 April 2017. This cannot be recorded in a structured manner in the verstrekkingsverzoek, but is included in the explanation (as free text).
Discontinuation of medication by third parties
Medication can be discontinued by the prescriber himself (see paragraph 4.1.13) but also by another prescriber. For example, the specialist can end medicatieafspraken made by a general practitioner that are up to date according to the information system, but that turn out not to be, for example after medication verification. When a health professional discontinues medication, he creates a new stop-MA. The health professional cannot modify someone else’s medicatieafspraak, only create a stop-MA. He therefore actively informs the health professional of the original medicatieafspraak about this change. The health professional of the original medicatieafspraak modifies the end date in the existing medicatieafspraak. If required, this may also be automated by the information system. This prevents the second health professional from not having to produce data after a period of time because the production period has expired (also no stop-MA), while the health professional of the original medicatieafspraak continues to produce it for no reason (without an end date).
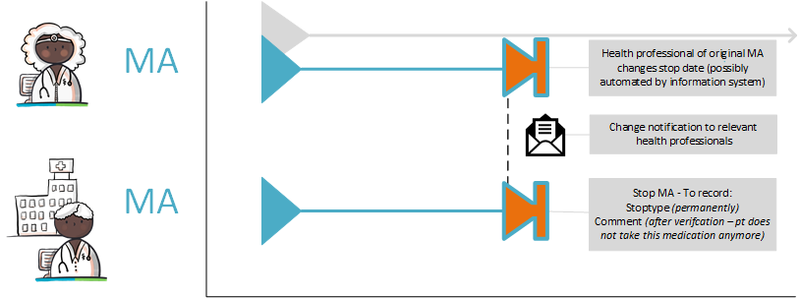
Two PRKs in a single medicamenteuze behandeling
In certain circumstances, the pharmacy is allowed to select a different PRK for the toedieningsafspraak than is indicated in the medicatieafspraak made by a prescriber. If for instance a failure of the prescriber’s information system would result in only the communication of the toedieningsafspraak, a subsequent prescriber will only have this toedieningsafspraak with the new PRK. The next prescriber will then probably assume that this PRK is also the PRK of the medicatieafspraak. A modification of the medicatieafspraak will then also result in a stop-MA (without referring to the original MA) and a new medicatieafspraak on the basis of this PRK. This means that there are now two medicatieafspraken with different PRKs under the same medicamenteuze behandeling. These two medicatieafspraken continue to exist. After any information system failure, (the information system of) the prescriber must check whether there have been changes and implement them, if necessary (see also paragraph 4.1.24).
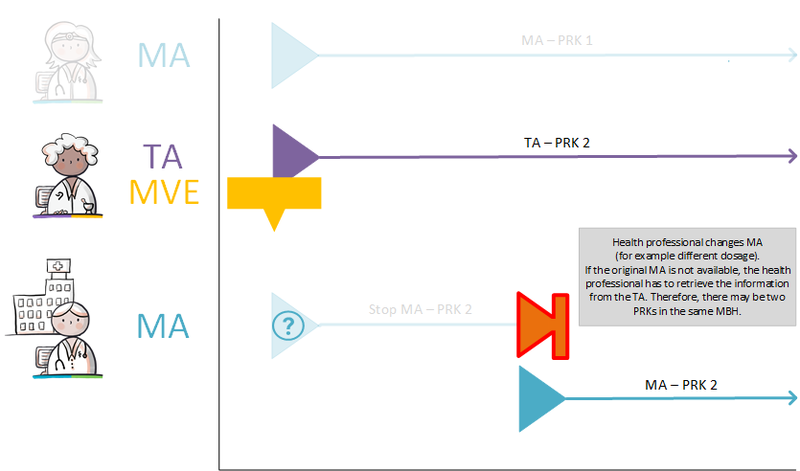
Creating a medicatieafspraak after the fact
In emergency situations, for example, it may happen that the medicatieafspraak is only created after the medication has been dispensed or administered. This may lead to conflicting medicatieafspraken. This means that one or more medicatieafspraken must be discontinued in order to prevent the occurrence of parallel medicatieafspraken. The only exception for parallel medicatieafspraken is described in paragraph 1.3.3.
Single medication use
For medication meant for single use, only an end date is included in the period of medication use. An example would be a vaccination (for example, a tetanus shot) or a situation in which a patient should take a medication just once, for example 3 weeks before he leaves on holiday.
Provisional and final medication order
The prescriber at the clinic prescribes medication. This is recorded in a provisional medication order (the medicatieafspraak). The hospital pharmacist verifies this order and records it as a final medication order (the toedieningsafspraak). See also [3].
Inadvertently ‘outstanding’ medication or 'orphans'
In a transitional situation and in a situation where not every XIS is connected, medicatieafspraken for the same treatment may exist that have been created by different information systems under different medicamenteuze behandelings. These medicatieafspraken should ideally be combined in a single medicamenteuze behandeling. This particularly applies to medication with an open end date, as this medication may have been discontinued under a different medicamenteuze behandeling. The medicatieafspraak therefore remains open, resulting in continued medicatieverstrekking. This is a so-called ‘orphan’ (a building block that has been registered, but in which, at a certain point, the health professional no longer has an active role. However, his information system continues to provide the building block, even if it has already been discontinued elsewhere). For medication with an end date, this problem will solve itself. Therefore, it is not necessary to take any action. When medication verification or medication assessment reveals that the medication is inappropriately still ‘open’, a stop-MA is created under the same medicamenteuze behandeling. The original prescriber is actively informed about this stop-MA and enters an end date for the medicatieafspraak in his own information system. Depending on the supplier and the client’s wishes, setting this end date can be done automatically or through an intervention of the prescriber. See also paragraph 4.1.24 and paragraph 4.1.37.
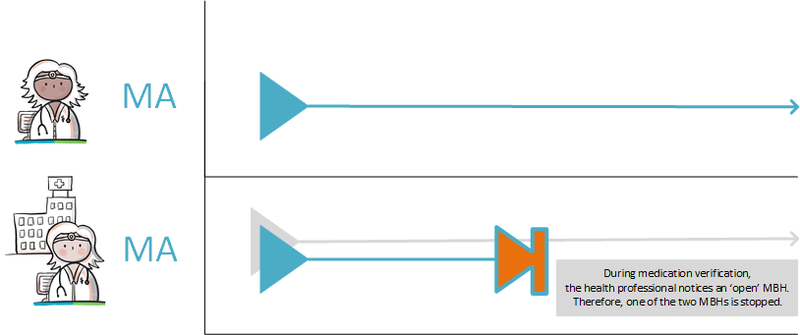
Missing digital medicatieafspraak at admission
If no digital medicatieafspraak is available when medication verification is carried out, a new medicamenteuze behandeling is started by recording medicatiegebruik. When this medicatiegebruik has to be discontinued, a stop-medicatieafspraak is created under the same medicamenteuze behandeling that refers to the recorded medicatiegebruik. If possible, the original prescriber (for example, general practitioner) and the pharmacy are actively informed about the discontinuation of this medication.
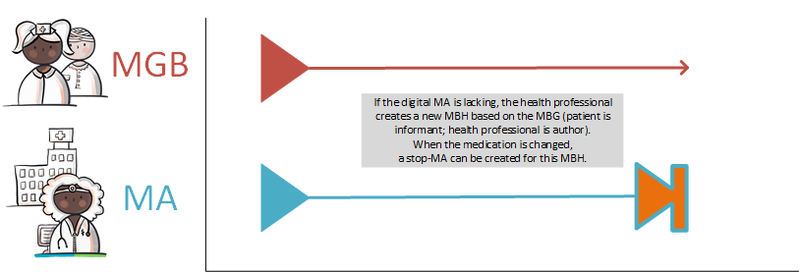
Own articles (90 million numbers)
It is possible to exchange a 90 million number. The condition is that within an organization a 90 million number once created may never be modified. During the exchange, the root OID (unique technical identification of the organization) in combination with the 90 million number ensures that it is unique compared to all 90 million numbers form other organizations. The substances that make up this article are included as ingredients in the medicatieafspraak (at least one ingredient), as is the case with magistrals.
Dosing with minimum interval
Medication can be prescribed at a fixed interval (for example: 2 tables every 6 hours). Prescribing medication with a minimum interval should be partly in free text. For example, if needed, a maximum of 3 times a day, a minimum of 6 hours between the intake moments: if needed, a maximum of 3 times a day is recorded in the standard way. The section “at least 6 hours between intake times” is recorded in free text as an Additional Instruction.
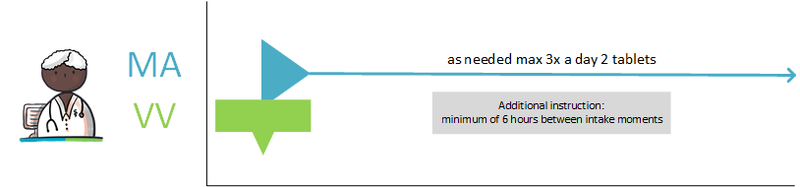
Verstrekkingsverzoek with number of repetitions
The prescriber makes a verstrekkingsverzoek with a medicatieafspraak in which she enters the number of repetitions. The prescriber hereby indicates to the provider that it may make additional provisions. The number of repetitions in combination with the quantity to be dispensed results in: (Number of repetitions + 1) X quantity to be dispensed. In case of “number of repetitions =3” and “quantity to be supplied = 30 pieces” the provider may provide 4 times 30 pieces (total 120 pieces). The number of repetitions in combination with the duration of medication usage period results in: (number of repetitions + 1) x duration of medication use. The amount to be dispensed depends on the dosage. In case of “dosage = 3 x per day 2 pieces”, and “Duration of medication usage period = 30 days” the provider may provide 180 pieces 4 times (6 pieces per day X 30 days = 180, 4 times = a total of 720 pieces).
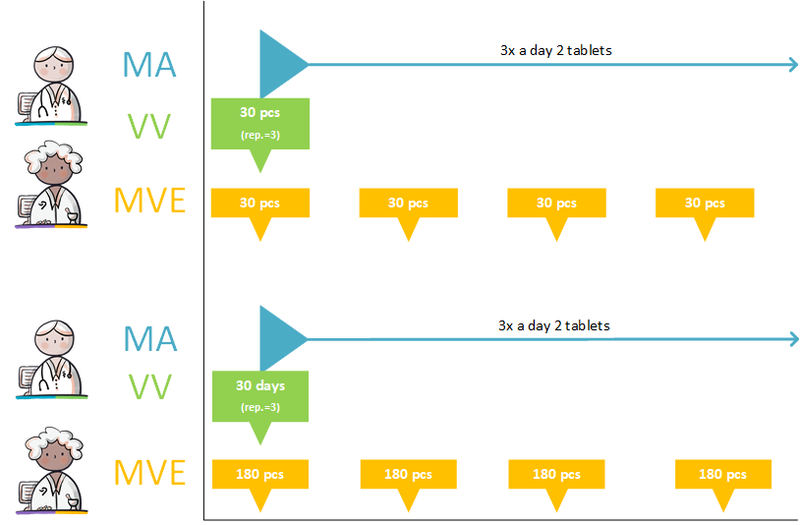
Prescribing non-medicines
Non medicines can be prescribed from the G-standard at HPK level. For example, this could be an inhaler (Aerochamber, HPK 1915185) as an aid to prescribed aerosols. Non-medicinal products are not applicable for the medication overview or medication monitoring.
Send renal function value in the prescription
A) Renal function value with a new prescription:
For the prevention of stroke, a patient (male, 65 years old) is prescribed Dabigatran for the first time. The patient has renal impairment and the prescriber has a recent renal function value available. Based on this renal function value, the prescriber deviates from the standard dose and makes a medicatieafspraak with a lower dosage of Dabigatran twice a day 1 capsule of 110 mg, starting from January 15, 2020. The prescriber also makes a verstrekkingsverzoek and sends the prescription (medicatieafspraak, verstrekkingsverzoek and the laboratory result of the renal function value) to the pharmacist.
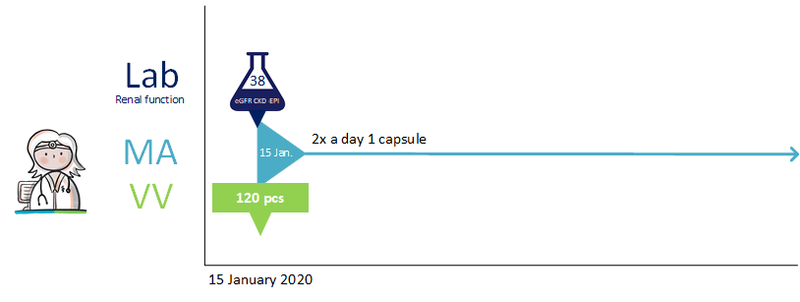
B) Renal function value reason for a change:
A 70-year-old man was prescribed 1 tablet of metformin 500 mg 3 times a day a few days ago (medicatie afspraak and verstrekkingsverzoek). At the same time, the doctor initiated a blood test to determine the renal function value of the patient. The value was not yet known at the time of prescription.
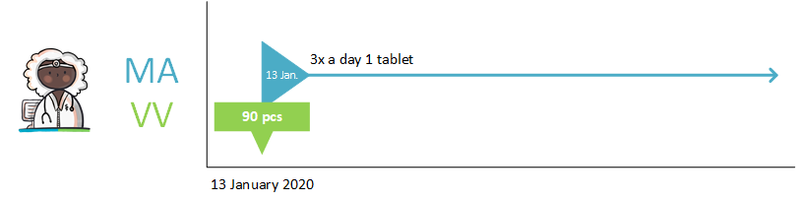
The blood test shows that renal function is impaired and gives reason to adjust the medicatieafspraak. The prescriber adjusts the dose on January 17, 2020 to 2 tablets metformin 500 mg twice a day and discuss this with the patient. The new prescription (the 'technical' stop medicatieafspraak, new medicatieafsrpaak and the laboratory result of the renal function value) is sent to the pharmacy by the prescriber. Because the patient had already collected the medication and therefore has enough stock, no new verstrekkingsverzoek is sent along.
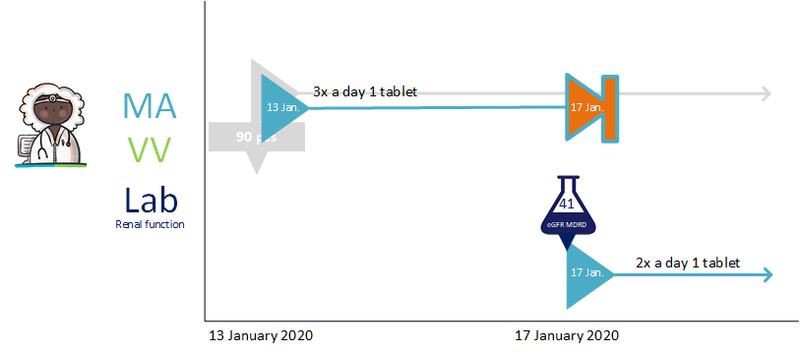
C) Renal function value due to drug:
The patient is prescribed Nitrofurantoin 100 mg, 1 capsule twice daily as a 5-day course due to a bladder infection and must start immediately on January 6, 2020. With this drug, the renal function value (if known and not older than 13 months) needs to be sent along. The prescriber has a renal function value available for this patient, but this does not lead to an adjusted dosage. The prescriber sends the prescription (medicatieafspraak, verstrekkingsverzoek and the laboratory result of the renal function value) to the pharmacist.
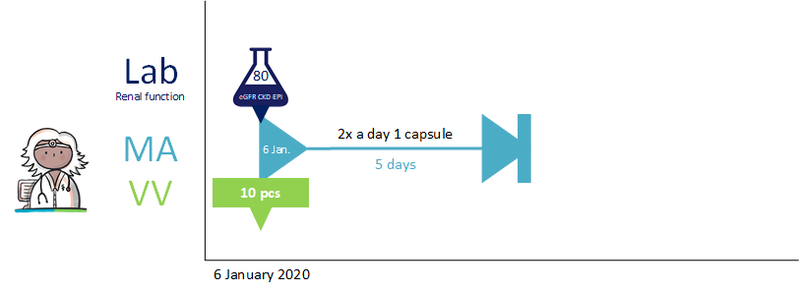
Cancelling a prescription that was sent earlier
A patient visits the general practitioner (GP) one morning because of stomach complaints. The GP prescribes pantoprazole and sends the prescription (MA + VV1) to the preferred pharmacy as known to him. In the afternoon the patient calls the GP. He hasn’t picked up the prescription yet and he would like to pick it up at another pharmacy due to a recent move, but forgot to ask this morning. The GP resends the prescription to the old pharmacy, but the VV in the newly send prescription contains a canceled indicator (MA + VV1 “cancelled”), so the pharmacist knows he should not dispense on this prescription. The prescriber then sends a new prescription (MA + VV2) to the patient's new pharmacy. The patient can pick up the medication there.
In medication building blocks it looks as follows:
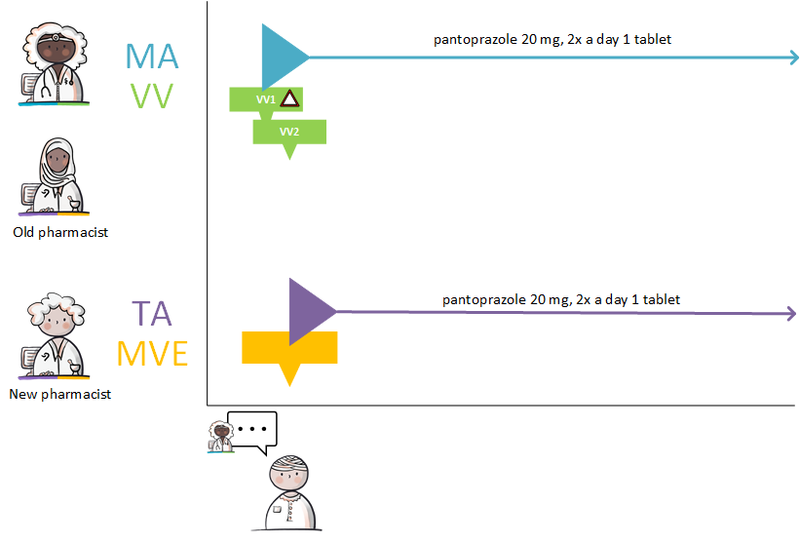
The transactions look as follows:
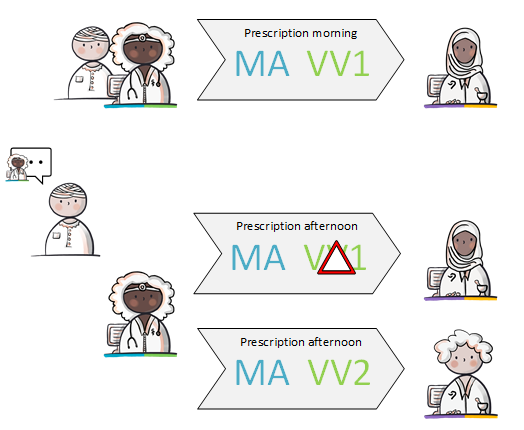
Modification of someone else's medicatieafspraak
A patient takes 40 mg Lisinopril once a day because of hypertension. This medicatieafspraak was previously made by the GP with the patient. The patient is admitted to the A&E department because of a fall due to dizziness. The specialist notices hypotension and decides to reduce the dose of Lisinopril to 20 mg once a day. The specialist then stops the current medicatieafspraak and starts the new medicatieafspraak with a lower dose. With this he changes the medicationafspraak with the patient. The specialist informs the original prescriber (the general practitioner) about this with a stop medicatieafspraak and the new medicatieafspraak.
The prescriber of the original medicatieafspraak adjusts the stop date in the original medicatieafspraak. If desired, this can also be automated by the information system. This prevents the second care provider no longer having to provide data over time because the delivery period has expired (including no stop MA) while the care provider of the original medication appointment continues to provide this incorrectly (without a stop date).
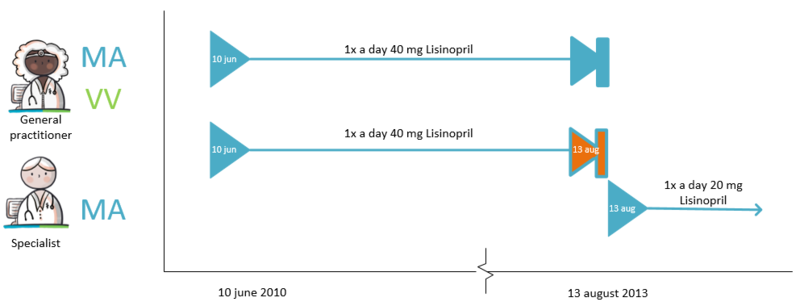
Setting up a variable dosing regimen (WDS)
The prescriber prescribes anticoagulants and states in the MA that the medication is used as recorded by the schedule of the thrombosis service (‘gebruik volgens schema trombosedienst). The prescriber sends a VV to the pharmacist and the patient is registered with the thrombosis service. In order to bridge the period until thrombosis care can take over, the prescriber creates a WDS for the first period. After registration, the thrombosis service creates a dosing regimen that overwrites or succeeds the previous regimen. From this moment on, the thrombosis service takes over creating the dosing regimen from the original prescriber.
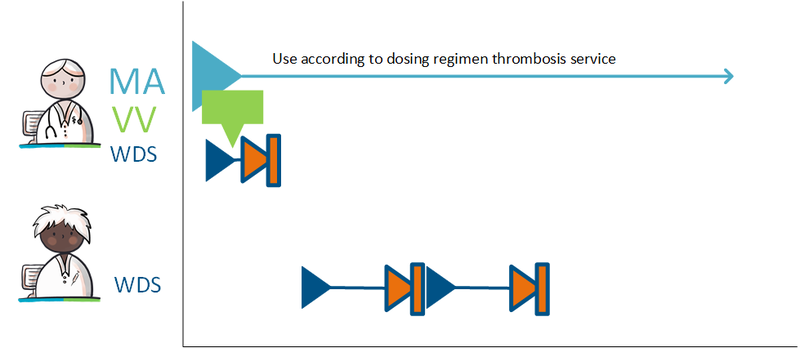
Changing a variable dosing regimen (WDS) during period of use
It could be necessary to revise the dosing regimen from a WDS before the scheduled stop date. For example, when the patient unexpectedly has to undergo minor surgery. In this case, the prescriber stops the current WDS with a technical stop (not visible to the user) and creates a new WDS with a few 0 doses the days before the procedure. In the meantime, the MA continues as usual.
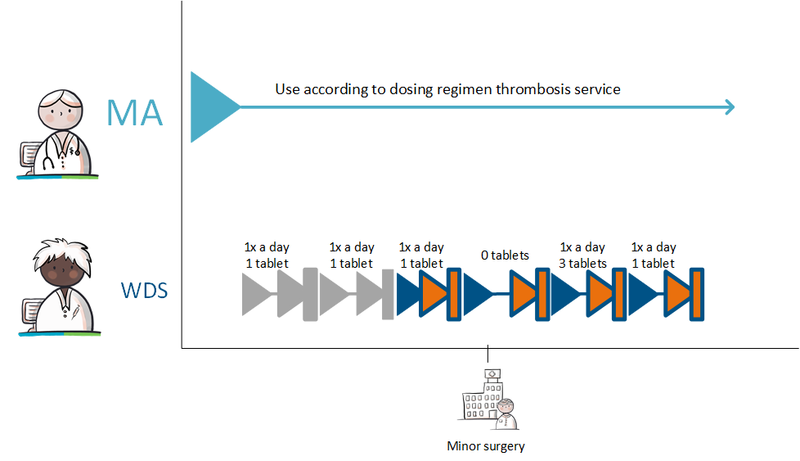
Stopping medication with a variable dosing regimen (WDS)
When the patient has to (temporarily) stop using anticoagulants completely, this is recorded at the level of the MA. The thrombosis physician creates a stop-MA. This also stops the underlying WDS and the associated TA. The original prescriber also processes the stop date in his MA.
If the anticoagulants needs to be restarted after several months, the original prescriber of the anticoagulant medication does this by following the process of starting a WDS. So by creating a new MA and a first WDS (see the use case Setting up a variable dosing regimen (WDS)).
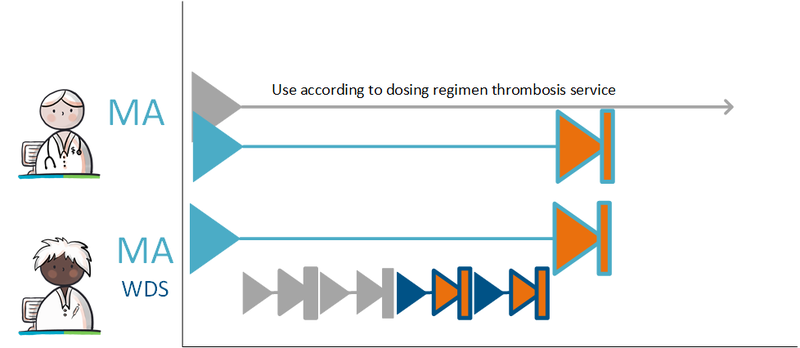
Use case, Dispense
New medicatieafspraak, medicatieverstrekking of the same product
The patient is at the pharmacist on 27 January 2013 to collect his medication. The pharmacist opens the file of the patient and verifies the medication file. It is a new medicatieafspraak for this patient. The relevant information of the prescribing physician is displayed on the screen of the pharmacist.
Medicatieafspraak: Nitrofurantoin CR capsule, 100 mg, 1 capsule 2x daily, from now on for 5 days.
Verstrekkingsverzoek: Nitrofurantoin CR capsule, 100 mg; 10 capsules.
The pharmacist selects a product on the basis of the medicatieafspraak and other factors (such as the pharmacist’s stock and the preference policy of the healthcare insurer):
FURABID CR CAPSULE, 100 MG. The pharmacist carries out medication monitoring using the information system, no (warning) signals are given.
The pharmacist carries out pharmaceutical care (compliance, education, et cetera) and agrees with the patient on how she will use the medication. This toedieningsafspraak on 27 January 2013 reads:
FURABID CR CAPSULE, 100MG; 1 capsule 2x daily; from now on for 5 days. The pharmacist provides the medication to the patient:
on 27 January 2013, FURABID CR CAPSULE, 100 MG; 10 capsules. The pharmacist actively informs the prescribing physician about the medicatieafspraak and the medicatieverstrekking.
The information system of the pharmacist makes the new toedieningsafspraak and medicatieverstrekking available to the other health professionals of this patient.
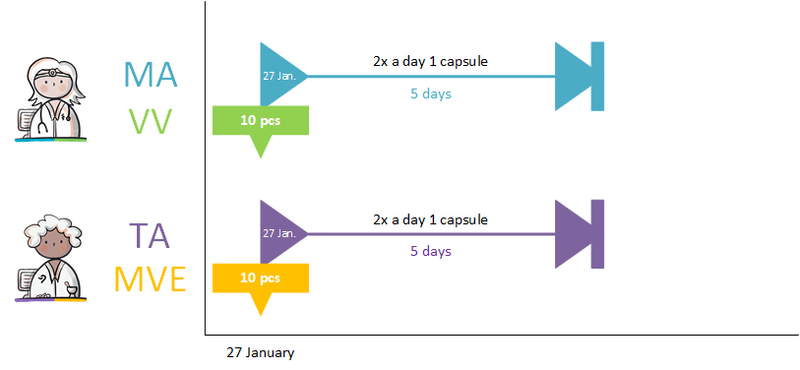
New medicatieafspraak, more precise product specification
Here, the difference when specifying the product is that the toedieningsafpsraak and also the medicatieverstrekking deviates from the medicatieafspraak the patient has made with the prescribing physician. The process is otherwise the same as described in the previous paragraph.
Example
Medicatieafspraak: Nitrofurantoin capsule mga 100 mg, 1 capsule 2 times a day from today for 5 days.
The pharmacist specifies a different product:
Nitrofurantoine MC Actavis capsule 50 mg.
The pharmacist agrees with the patient on how she will use the medication. This toedieningsafspraak on 27 January 2013 reads:
Nitrofurantoin MC CR capsule 50 mg, 1 capsule 2x daily, from now on for 5 days.
The pharmacist provides the medication to the patient:
on 27 January 2013, Nitrofurantoin MC Actavis capsule 50 mg; 20 pieces.
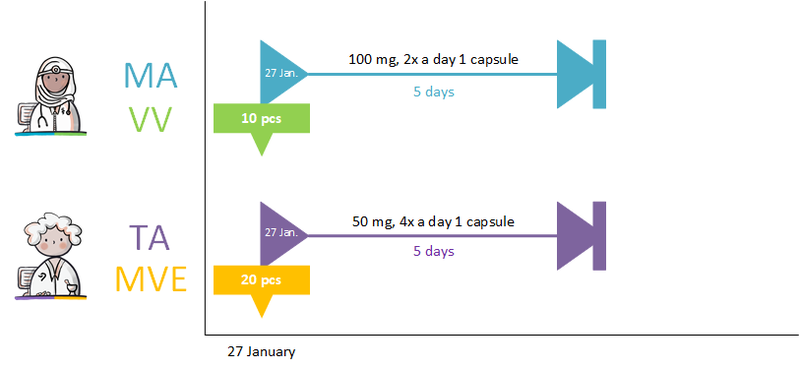
Existing toedieningsafspraak is adequate
The process of repeat medicatieverstrekking on the basis of an existing medicatieafspraak and possibly existing verstrekkingsverzoek and toedieningsafsrpaak occurs in case of repeat prescriptions and is described in paragraph 4.2.10, situation 2.
Medicatieafspraak wanted (informing the prescriber)
Example 1 - adjusting dosage:
For a 70-year-old patient with a creatinine clearance of 45 mL/min, the pharmacist enters a toedieningsafspraak for gabapentin tablets of 600 mg, 1 tablet 3x daily. Renal function has been recorded in the patient’s file at the pharmacy, and based on this a signal appears that the maximum dosage is 900 mg daily. The pharmacist sends the prescriber a proposed medicatieafspraak with an adjusted dosage of 300 mg, 3x daily, and the reason for this proposal (maximum dosage exceeded). The prescriber receives the proposed medicatieafspraak in his EVS. The prescriber sends an adjusted medicatieafspraak and possibly a reply proposed medication agreement (antwoord voorstel medicatieafspraak) back to the pharmacist.
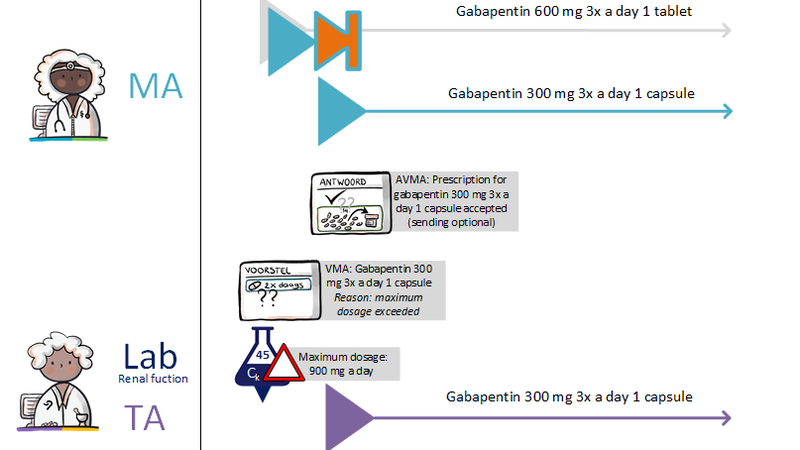
Example 2 – temporarily halting a medicinal product:
For a 50-year-old patient with on oral fluconazole as part of his current medication, the pharmacist enters a toedieningsafspraak for simvastatin. A message appears with the advice to consider temporarily halting simvastatin. The pharmacist can make a proposed medicatieafspraak for halting the simvastatin (stoptype ‘temporary’). See example 1 for further steps.
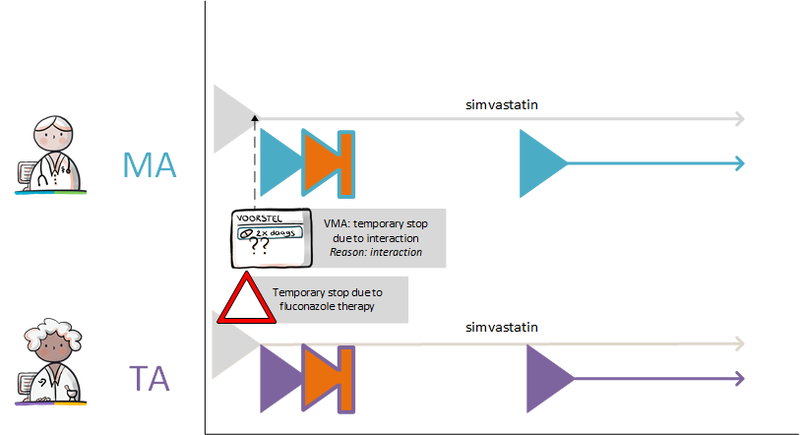
Example 3 – adding a medicinal product:
For a 55-year-old woman, the pharmacist enters a toedieningsafspraak for prednisone, 10 mg daily for three months. The patient is not using osteoporosis prophylaxis. A message appears with the advice to add a biphosphonate drug and to check whether the patient is taking calcium and vitamin D. If the patient is not, the pharmacist can make a proposed medicatieafspraak for these products. See example 1 for further steps.
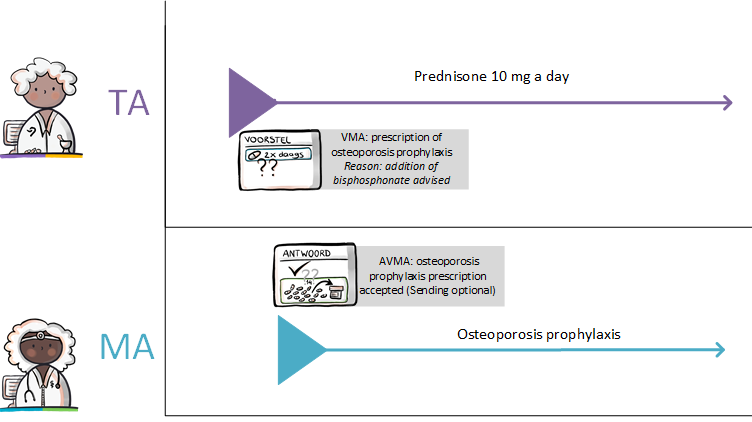
PLEASE NOTE: it will depend on the situation whether it is useful to create an automated proposal for adjustment/addition of a MA. If the advice is complex, it may still be useful to phone and to consult instead of sending a digital proposal.
The proposed medicatieafspraak may help the prescriber to enter this change in the system. The latter is important when medicatieafspraken are consulted, for example, by service observation general practitioners.
Request and dispense
When a medicatieafspraak and verstrekkingsverzoek arrives at the pharmacy, it is processed by the pharmacy information system as an intended medicatieverstrekking. This is called the request date, which is captured as the request date of the medication dispensing The date of dispensing is only registered at the moment of pick up or hand over of the medication. The pharmacist informs the prescriber about the handling of the prescription (toedieningsafspraak and/or medicatieverstrekking).
It is possible to create multiple dispensings under the same toedieningsafpsraak:
- A verstrekkingsverzoek comes in with a medicatieafspraak with a one-year use period
- There will be a first provision with a new toedieningsafspraak and a medication supply that is sufficient for, for example, a period of 4 months.
- The second dispensing is performed based on the existing medicatieafpsraak, verstrekkingsverzoek and toedieningsafspraak (no new prescription or new administration appointment is needed). The new second medication dispensing (with new write-up date and the pre-existing associated toedieningsafspraak) is sent to the prescriber when the dispensing date is registered.
Patient requests repeat prescription via physician (reactive repeat)
A physician has previously diagnosed hypertension in a patient (57 years old) and has prescribed the patient medication for this. The patient uses this medication and the medication is running out. The patient makes a request to repeat the medication, for example through the repeat line, by phone, by email to the practice, counter/boxes, website, app or portal of the physician. The physician approves the repeat. This leads to a new verstrekkingsverzoek.
For example:
The general practitioner makes, in the context of the medicatieafspraak of 30 March 2010:
- Nifedipine CR 30 mg; 1 tablet 1x daily, from 30 March 2010 for an indefinite period
on 13 April 2013 a verstrekkingsverzoek to a pharmacist chosen by the patient:
- Nifedipine, CR tablet, 30 mg; ‘for three months’.
In consultation with the patient, the prescriber can send the verstrekkingsverzoek with the corresponding medicatieafspraken and toedieningsafspraken as an order to a pharmacy of the patient’s choice. The prescriber also makes the new verstrekkingsverzoek available to fellow health professionals and the patient. The existing medicatieafspraken had already been made available.
The pharmacist receives the verstrekkingsverzoek together with the medicatieafspraak and processes it in accordance with the dispense process and starts with the pharmaceutical care process step.
When the patient, on the basis of a toedieningsafspraak or on the basis of his medicatiegebruik asks for a repeat prescription, and the medicatieafspraak is not digitally available, the physician creates a new medicatieafspraak based on this toedieningsafspraak or the patient’s medicatiegebruik of the medication. No reference is made to the existing medicatieafspraak in that case.
Patient requests repeat prescription via pharmacist (informing prescriber)
The patient requests a repeat medication from the pharmacist, for example through repeat line, by phone, email, counter/boxes, website, app or portal of the pharmacist.
The pharmacist sends a proposed verstrekkingsverzoek for authorisation to the prescriber. The physician evaluates the proposed verstrekkingsverzoek and approves it. He informs the pharmacist via the reply proposed verstrekkingsverzoek and sends a new verstrekkingsverzoek together with the current agreements to the pharmacist. The pharmacist continues the dispense process and starts with the pharmaceutical care process step.
Patiënt vraagt herhaalrecept via arts (reactief herhalen)
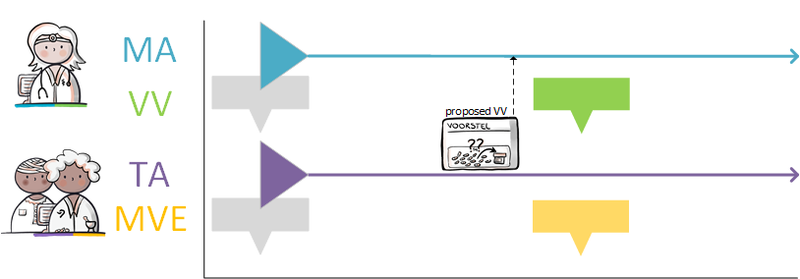
If the prescriber does not provide a verstrekkingsverzoek, he informs the pharmacist via the answer proposed verstrekkingsverzoek.
Proactive repeat prescription by pharmacist (informing prescriber)
The patient has signed up in the past for proactive repeats and the pharmacist has registered this in his proprietary pharmacist information system (PIS). The repeat module of this information system will generate a signal when a patient needs new medication. When a new verstrekkingsverzoek is needed, this is similar to the process described in the previous paragraph, only the trigger is not the patient but the pharmacist (or his information system). Proactive repeats are also used for GDS, see paragraph 4.2.11.
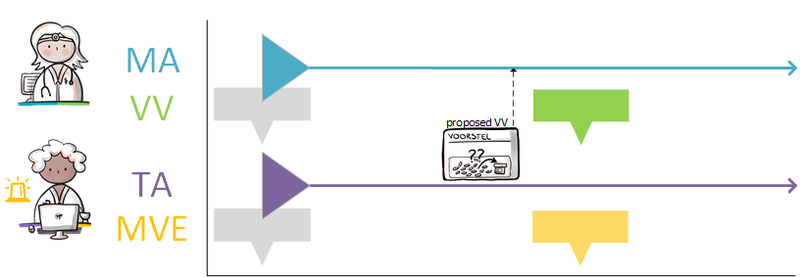
Dispense based on an existing verstrekkingsverzoek
The patient submits a repeat prescription to the pharmacist. In this case, it is a request for medicatieverstrekking on the basis of an existing, still valid, verstrekkingsverzoek. See paragraph 4.2.10 situation (3).
Splitting a prescription
The patient has been using the same medication for a number of years, which means that the medicatieafspraak can be created for an indefinite period. The toedieningsafspraak is also valid for an indefinite period. In such a case, the patient can be given a prescription for a year. This single year prescription will be split into several prescriptions by the pharmacist. These split prescriptions are commonly called ‘repeat prescriptions’, even though strictly speaking this is not true.
There are three possible situations:
1) The patient returns to his attending physician still suffering from the same symptoms. The prescriber prescribes the same medication (in the case of an expired medicatieafspraak, a new medicatieafspraak is created with a new verstrekkingsverzoek and in the case of a medicatieafspraak that is still valid, only a new verstrekkingsverzoek is created).
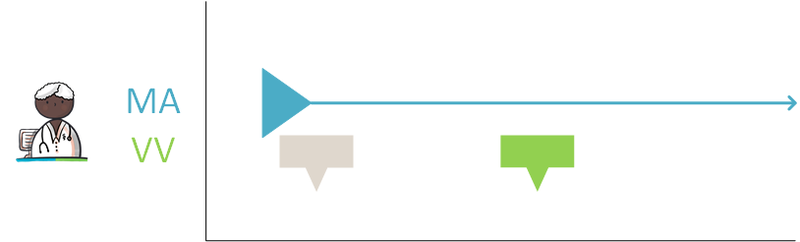
2) Prescriber and patient initially agree on 90 tablets with 3 repeat prescriptions. The patient receives the first 90 tablets. The next time the patient visits the pharmacist, a medicatieverstrekking is made under the existing agreements.
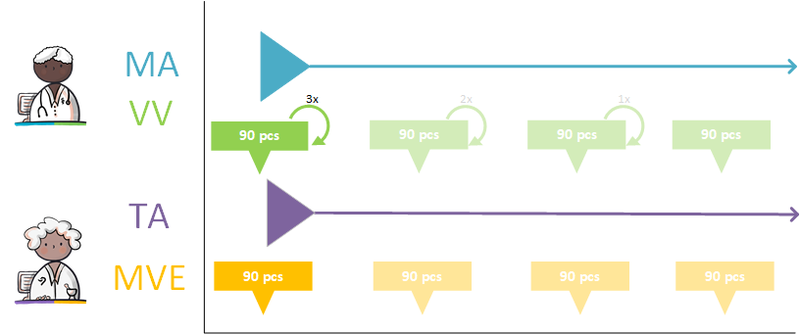
3) Prescriber and patient initially agree on 360 tablets for one year. The pharmacist splits this into 4 separate occasions of medicatieverstrekking. The patient is initially dispensed 90 tablets and without a new verstrekkingsverzoek, can go to the pharmacist 3 more times to pick up another 90 tablets each time.
Situation 3 best describes the way in which a pharmacist splits a year prescription.
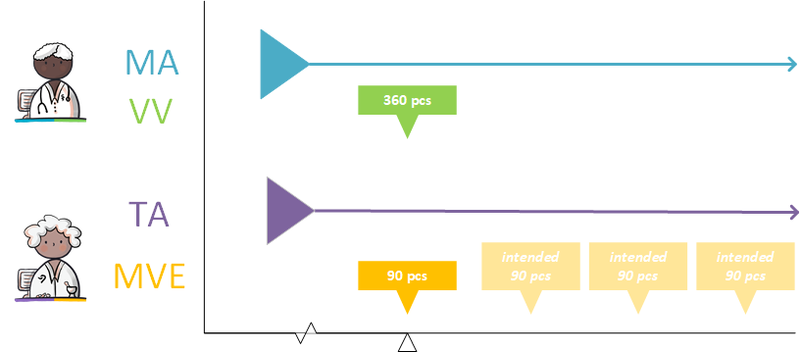
Starting and continuing a GDS
The following example shows the process for one medicinal product used by the patient for an indefinite period of time. The information system of the prescriber and the pharmacist contains (among other things) the following data on a patient:
- The medicatieafspraak of 2 January 2013 reads: Metoprolol, CR tablet, 100 mg (succinate); 1x tablet daily; from 2 January 2013 for an indefinite period.
- The toedieningsafspraak of 2 January 2013 reads: Metoprolol PCH retard, 100 CR tablets of 95 mg; 1x tablet 1x daily; from 2 January 2013 for an indefinite period.
This elderly patient is taking so much medication that he has problems overseeing it all: a potentially dangerous situation. On 6 February, the pharmacist and the prescriber agree that the medicatieverstrekking of medicinal products to this patient will take place via GDS.
The GDS is started: the pharmacist sends a proposed verstrekkingsverzoek (a similar event to the current combined prescription or authorisation form).
The proposed verstrekkingsverzoek of 6 February reads:
- Verstrekkingsverzoek for the medicatieafspraak of 2 January 2013: Metoprolol, CR tablet, 100 mg (succinate), and a period of use to ensure enough stock up to and including 1 May 2013.
The pharmacist sends the proposal to the prescriber. The prescriber approves the proposal on February 6 and sends it unchanged to the pharmacist as a verstrekkingsverzoek. The GDS indicator is switched on in the information system of the prescriber as well as in the pharmacist’s information system.
The pharmacist and the patient select a partial duration for the product. This toedieningsafspraak reads as follows:
- On 7 February it was agreed: Metoprolol PCH retard, 100 CR tablets of 95 mg, 1 tablet in the morning, from 11 February (week 6), for an indefinite period.
Every second week, the pharmacist dispenses a Baxter medication roll containing (among other things) the metoprolol:
- Medicatieverstrekking 1: The pharmacist dispenses, on Friday, 8 February 2013: 14 tablets, scheduled for weeks 6 and 7 (a period of use of 2 weeks).
- Medicatieverstrekking 2: The pharmacist dispenses, on Friday, 22 February: 14 tablets, scheduled for weeks 8 and 9.
- Medicatieverstrekking 3, 4, 5, etc.
With each medicatieverstrekking, it is indicated that medicatieverstrekking was carried out via GDS.
After three months, there is a new proposed verstrekkingsverzoek from the pharmacy to the prescriber, etc.
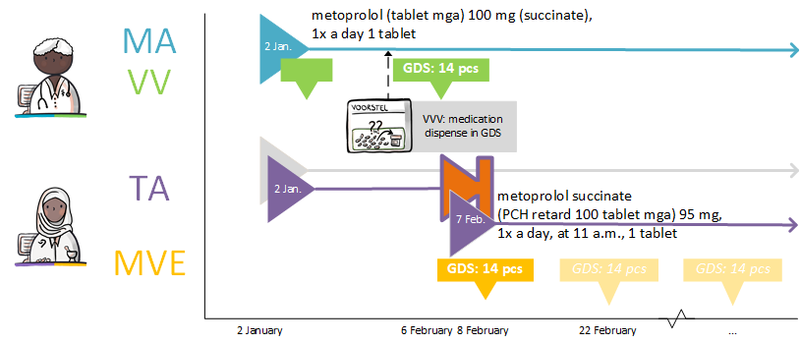
Consideration
- The application of a proposed verstrekkingsverzoek instead of an authorisation form makes its meaning unambiguous.
- In the current situation, toedieningsafspraken and medicatieverstrekkingen are registered as a single dispense. This makes it difficult to find useful information in the large number of messages. By dividing the dispense order into two building blocks, the overview is easier to understand: there are no new medicatieafspraken and only one new toedieningsafpsraak is created. There are no hidden surprises in the remaining logistical data and the prescriber does not have to monitor the multiple medicatieverstrekkingen.
- This way of registering and exchanging prevents double registration, and errors caused by this.
- The toedieningsafspraken are the basis for the administration list and checklists of nursing homes and care homes. Signing them corresponds to the administration(s).
The pharmacist changes commercial product
This scenario builds on the previous scenario: after half a year a different commercial product is agreed with the patient (toedieningsafspraak), because the original product is not available. No new medicatieafspraak is made, as there is no change needed on that level. The toedieningsafspraak is changed:
- On 1 July it was agreed: Metoprolol Sandoz ret, 100 CR tablets, 95 mg, 1 tablet in the morning; from 3 July, for an indefinite period. Reason: not available.
The toedieningsafspraak is a modification of the toedieningsafspraak of 7 February, which stops on 2 July (11:59 p.m.) and is an actual handling of the medicatieafspraak of 2 January. The pharmacist informs the general practitioner about this.
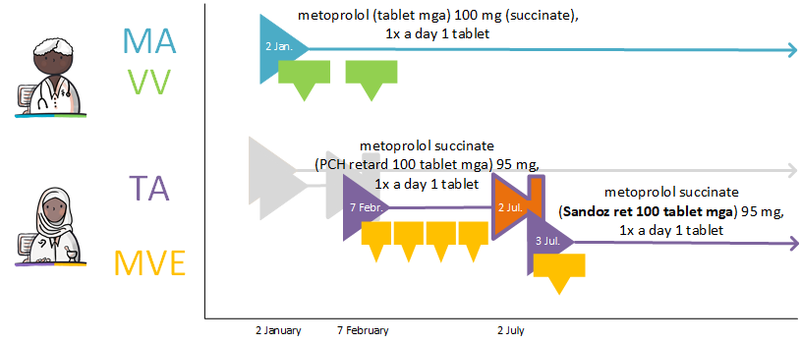
Consideration
This change in the actual handling of the medicatieafspraak using a toedieningsafspraak, changes nothing to the validity of the medicatieafspraak itself, improving the overall view for all parties. In addition, since the pharmacist records and communicates the reason for the adjustment, the prescriber is better able to assess whether the information is relevant.
Adding medication to a GDS
When new medication is added to an existing GDS, the prescriber may opt for immediate addition to the current roll (Change in GDS immediately) or for addition starting from the next roll (Change in GDS per change of roll). The prescriber indicates this in the medicatieafspraak (Additional information).
If ‘Change in GDS immediately’ is chosen, the pharmacist will first dispense the medication separately, in addition to the existing GDS. This means that a toedieningsafspraak is created by the pharmacist for the bridging period. Starting with the new roll, the new medication is added to the roll. Starting with the start date of the new roll, a toedieningsafspraak can be made by the pharmacists with specific administration times. If the ‘bridging-toedieningsafspraak’ already includes the correct administration times, no new toedieningsafspraak is created at the date of inclusion in the roll.
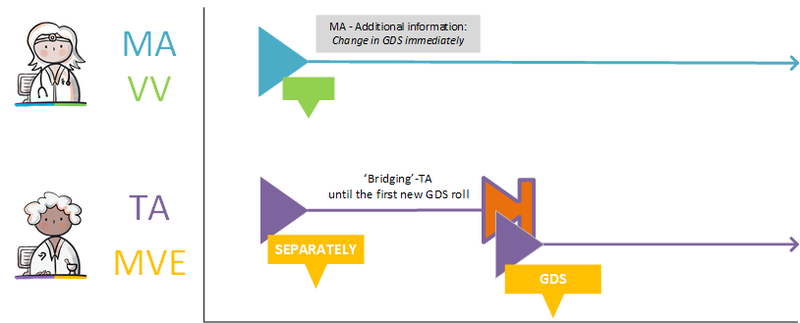
If 'Change in GDS per change of roll' is chosen, the pharmacist will start to include the medication in the roll on the start date of the first new roll. In this case, a toedieningsafspraak for the bridging period is not required.
Discontinuing medication in a GDS
When medication is discontinued, the prescriber creates a discontinuation-medicatieafspraak. In accordance with the process, the pharmacist is informed about this stop-MA and can adjust the medicatieverstrekking accordingly. The pharmacist will create a staken toedieningsafspraak . See paragraphs 2.2.5.3 and 2.3.6.3.
GDS supplier supplies other commercial product
It is possible that a GDS supplier delivers a different commercial product than that which is included in the toedieningsafspraak by the pharmacy. This is therefore a change in the HPK which results in a changed toedieningsafpsraak. This may only be afterwards, after feedback from the GDS supplier of the filling details.
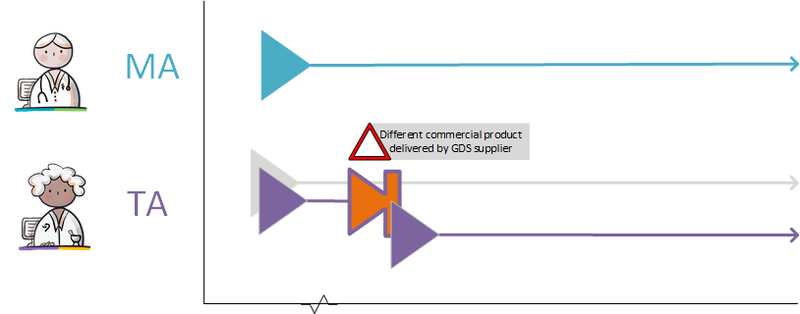
Parallel administration agreements with GDS- and non-GDS-dispense
A patient takes two to three times daily 20 mg pantoprazole because of gastric complaints. The first two tablets are fixed dosages, which are dispensed in GDS. The third tablet, which is prescribed as needed, is dispensed separately. At a certain moment, Sandoz is unable to supply the pantoprazole 20 mg for separate dispenses. Therefore, the pharmacist decides to dispense from Apotex. He stops the current TA and splits the TA in two TA’s, both with the same start date/time. The first TA concerns the fixed dosage in GDS, the second TA involves the as needed tablet.
NB. Currently, there is no relationship between medication dispense and TA. Therefore, it is only possible to retrieve which TA is associated with the medication dispense by assessment of the data content. It is evaluated whether the functional design should be adjusted for this use case in the future.
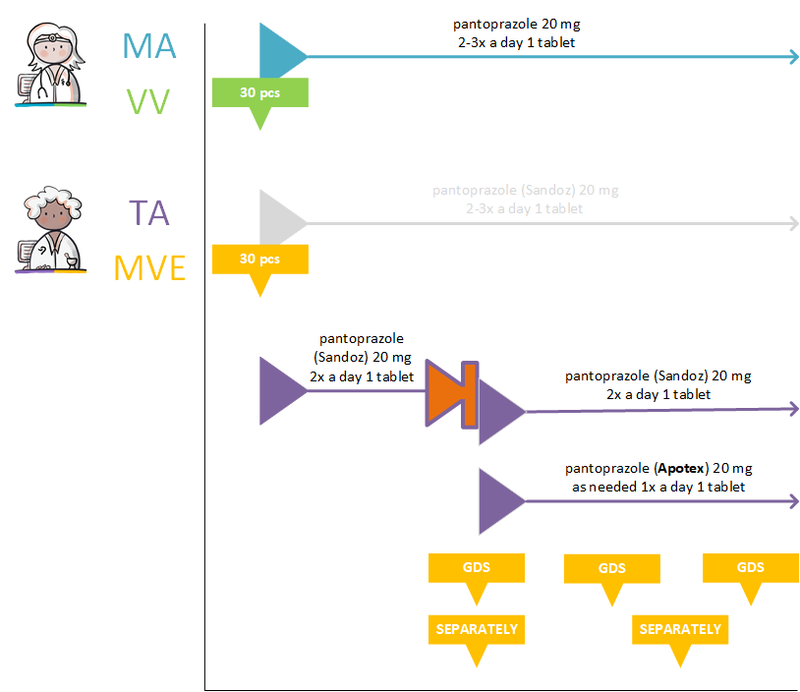
Handling a stop medicatieafspraak
When the prescriber agrees to discontinue medication, a stop medicatieafspraak is created. This stop-MA this processed by the pharmacist by discontinuing the corresponding toedieningsafspraak.
Medication dispense with someone else’s administration agreement
A patient takes two times daily 20 mg pantoprazole because of gastric complaints. During the holidays the patient notices that he has forgotten to bring his medication. He visits the after hours general practice clinic and asks for extra tablets for during his holidays. The after hours physician sends a VV to the after hours pharmacy, using an existing MA.
The after hours pharmacist reads the medication prescription and creates an MVE, using the existing TA, for the ten tablets that the patient needs. The pharmacist notifies the after hours physician about the medication dispense, and sends a copy of the original TA.
NB. Although the original prescriber and supplier do not receive an automatic notification, the data will be made available to them.
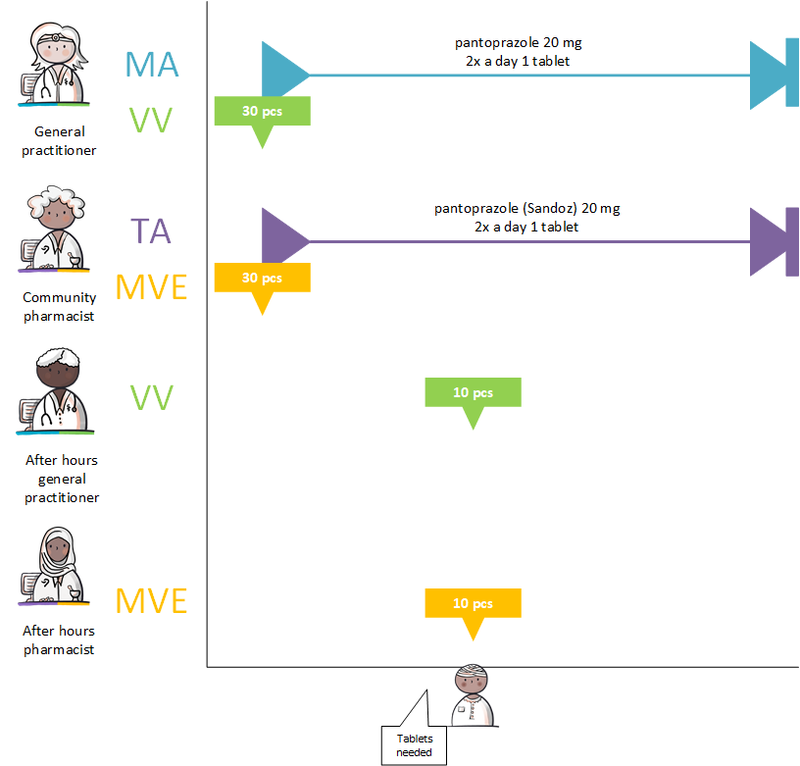
Modification of someone else’s administration agreement
A patient takes two times daily 20 mg pantoprazole because of gastric complaints. During the holidays the patient notices that he has forgotten to bring his medication. He visits the after hours general practice clinic and asks for extra tablets for during his holidays. The after hours physician sends a VV to the after hours pharmacy, using an existing MA.
The after hours pharmacist notices that they have no pantoprazole from Sandoz in stock, but they do have pantoprazole from Apotex. Therefore, she stops the current TA, starts a new TA for the Apotex, and creates an MVE for the ten tablets from Apotex. The after hours pharmacist notifies the original pharmacist and, subsequently, the original pharmacist processes the stop-TA.
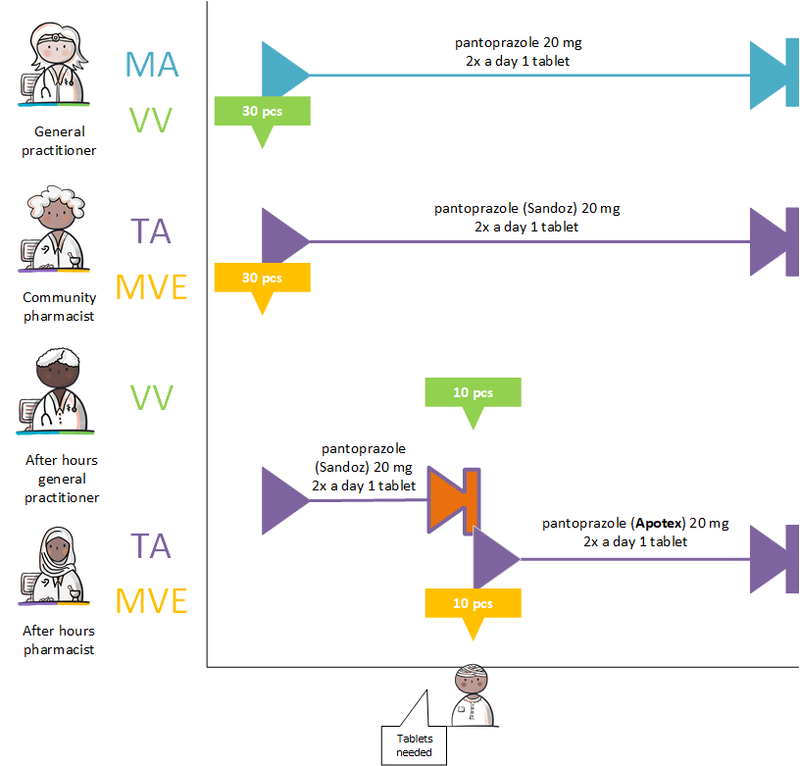
Use cases, Administer
Creating an administration list
When an administration list has to be created, for example when a patient starts to receive medication administered by a (professional) administrator, or when GDS medication or non-GDS medication is started, an intake is planned with the patient and the prescriber, pharmacist or administrator (the exact process may differ across institutions). Then, the prescriber, pharmacist, administrator and/or the patient consult on the administration list (which medication is recorded on the administration list, which (guide) administration times are suitable). The pharmacist enters the (guide) administration times in the TA and (actively) makes the relevant medication data available for the administrator and/or the patient.
Exact administration times required
When exact administration times are required, for example because of medication interactions, the prescriber or the pharmacist can indicate that an exact administration time is required (the administration time is not a guideline but denotes an exact time). The prescriber or pharmacist can register an exact administration time by indicating that the administration time is not flexible. By default, administration times are flexible. Exact administration times should be presented on the administration list, to ensure that the administrator is informed that it is not allowed to deviate from the entered administration time.
Missing (guide) administration times
When (guide) administration times are missing in the MA and TA, an administrator will contact the prescriber or pharmacist to retrieve the (guide) administration time. This is not supported digitally in this information standard.
Non-GDS medication as needed
Non-GDS medication as needed is included in the administration list, but is not listed according to the administration moment and/or administration time, because this information is not available beforehand. If relevant (for example because of drug interactions), (guide) administration times can be entered. See the following use cases for practical examples: 4.1.4 Medication as needed, 4.1.5 Course of treatment as needed starting in future, 4.1.6 Two dosages of the same medication at the same time, 4.1.7 The same medicinal product with different strengths at the same time.
Medication supply by multiple pharmacies
Multiple pharmacies may be involved in the medication supply for one patient, for example in the following situations: medication supply by an outpatient pharmacy, in addition to supplies by a community pharmacy or supplies of add-on medication by a hospital pharmacy. In the case of medication supply by multiple pharmacies, each pharmacist is responsible for the medication data that is required for the administration list, including the (guide) administration times in the TA. It is the responsibility of the prescriber or pharmacist who has made the most recent medication adjustments to determine whether an addition/modification is appropriate with regard to the total of (known) medication data; this concerns both medication monitoring and (guide) administration times. The medication data is (actively) made available, in order to enable that all medication data from different pharmacies are compiled in one administration list.
Change in GDS from the next supply or immediately
If GDS medication is altered, it depends on the situation whether this change should occur immediately or from the next supply. The prescriber can indicate in the MA when the change in GDS medication should be implemented. An immediate modification should be immediately shown on the administration list, to ensure that the (professional) administrator administers the correct (amount of) medication. In contrast, a modification that should be implemented from the next supply, should not be processed immediately in the administration list, since previous agreements remain valid until the next supply. Thus, the administration list should not be modified until the change in GDS modification has become effective.
See also use case 4.2.13 Adding medication to a GDS.
Increasing dosage of GDS in new MBH
A patient is administered 1 tablet propranolol 40 mg 1 time a day. Due to insufficient effects, the prescriber decides to increase the dosage to 80 mg 1 time a day. A new MBH is created for this MA, as there is a change on prescription level. However, the patient still has the 40 mg tablets in the GDS medication roll for the coming days. Therefore, the pharmacists decides to supplement the GDS medication with the administration of 1 tablet propranolol 40 mg, separately from the GDS package, in addition to the tablet in the GDS package, until the next GDS medication roll change in 5 days. The pharmacists creates two TAs: one for the previous GDS medication and one for tiding over the period with medication dispense separately from GDS. In the new GDS supply (medication roll change), the 80 mg tablets are incorporated in this new GDS package.
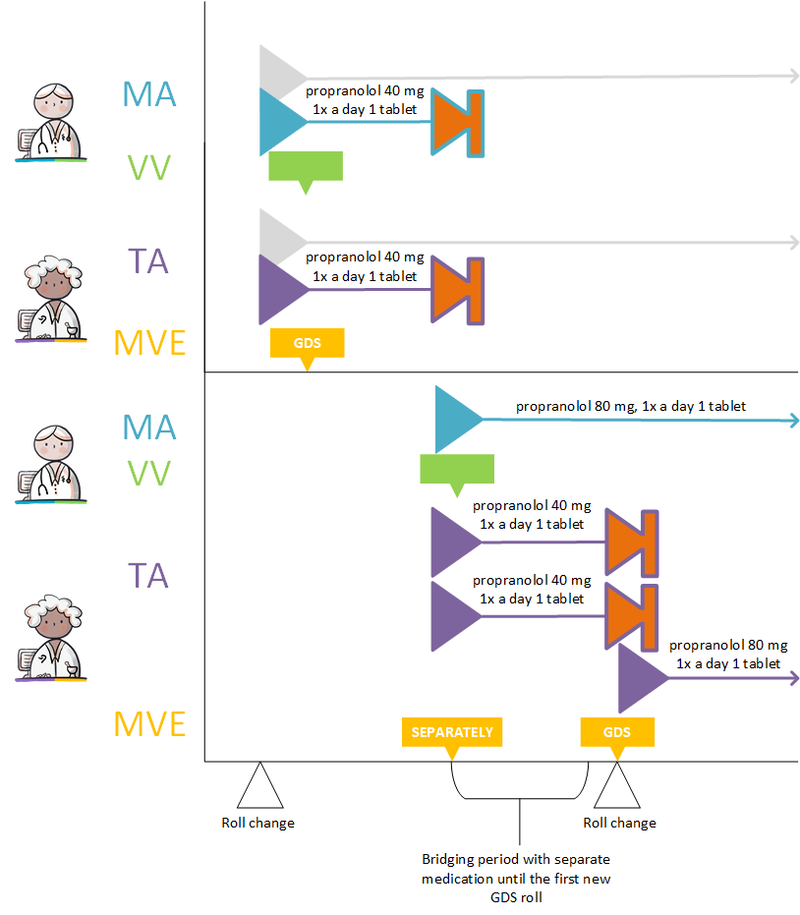
Decreasing dosage of GDS in new MBH
A patient is administered 1 tablet propranolol 80 mg 1 time a day. Due to a too strong effect, the prescriber decides to reduce the dosage to 40 mg 1 time a day. A new MBH is created for this MA. The patient still has 80 mg tablets in the GDS medication roll for several days. Because the administrator is not allowed to divide the tablets into halves, the supplier decides that the remaining GDS packages should be collected the same day and be replaced by a new GDS medication roll with 40 mg tablets.
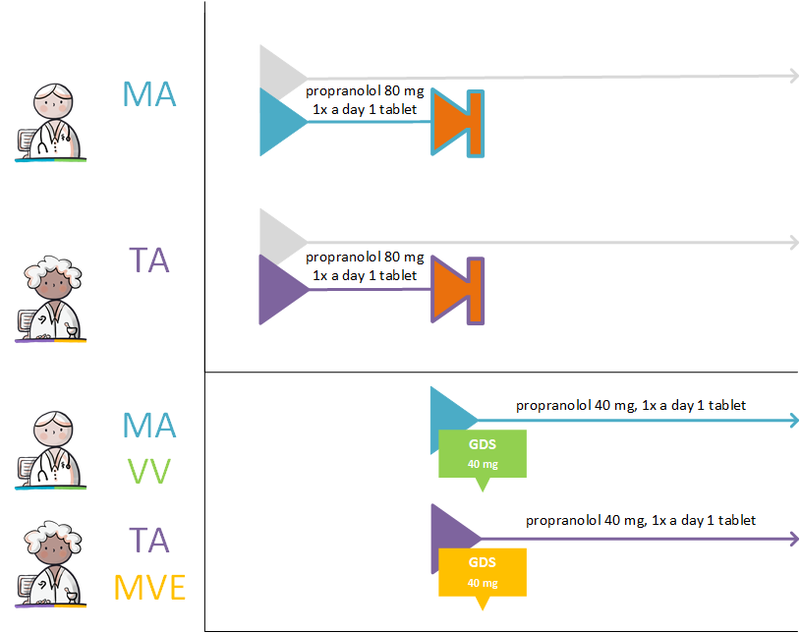
Change processed by the pharmacist
A change by the prescriber, such as starting new medication, adjusting the dosage, (temporarily) halting medication, is recorded by the prescriber in the MA and/or the VV. Then, the pharmacists processes this change in the TA and/or the MVE. This may be done during the opening hours of the community pharmacist, or after hours when a medication dispense is needed. The after-hours pharmacist dispenses the medication; in this case, multiple pharmacists are supplying medication (see also paragraph 4.3.9). The medication data of the pharmacist(s) and the prescriber(s) are (actively) made available by the prescriber and the after-hours pharmacist to the administrator for the administration list.
Change not processed by the pharmacist
When medication is changed by a prescriber after the opening hours of the community pharmacist and medication dispense is not needed, no pharmacist is involved. In that case, the MA is decisive, and the prescriber (actively) makes the MA available. The administrator will be informed of the changed or stopped MA by the administration list; this MA overrules all medication building blocks within the same MBH. This situation also applies if the pharmacist has not processed the modification by the prescriber yet. The pharmacist is responsible for adjusting the TA as soon as possible.
Variable-dosing regimen
If a physician prescribes medication with a variable-dosing regimen, the dosing schedule of this medication may be specified by another physician. For example, this applies to thrombosis medication: the prescriber defines the therapeutic INR range (International Normalized Ratio, a measure for the coagulation time of blood), but refers the patient to the thrombosis service for defining the exact dosing schedule. The ‘trombosearts’ (physician affiliated to the thrombosis clinic, who is specialized in the management of anticoagulation therapy) enters the variable-dosing regimen in the medication building block WDS. The prescribing physician remains responsible for the supplies, the ‘trombosearts’ specifies the dosing regimen based on the therapeutic INR range. As the WDS is a separate medication building block, the medication overview remains unchanged (the MA is specified, not altered by the WDS). The WDS is used to compile the administration list, together with the medication data in the MA, TA, MVE and MTD.
Changing a variable-dosing regimen
If a dosing schedule is adjusted, the information system closes the current WDS, using a technical stop (not visible to the user). This new WDS is related to the MA and the previous WDS.
Stop of medication with variable-dosing regimen
Anticoagulation medication may be (temporality) halted for several reasons; for example, in the case of a medical procedure. Depending on the situation and based on professional judgment, the ‘trombosearts’ can choose one of the following two methods:
A. (temporarily) adjusting the medication regimen
In certain situations, the ‘trombosearts’ may choose to temporarily set the anticoagulation medication to zero, for example, in the period before the planned medical procedure. The MA (and, therefore, the treatment with anticoagulation medication) continues, but in the WDS, the dosage is temporarily adjusted to zero. In such situations, it is advisable to exchange the reason for the WDS change as well.
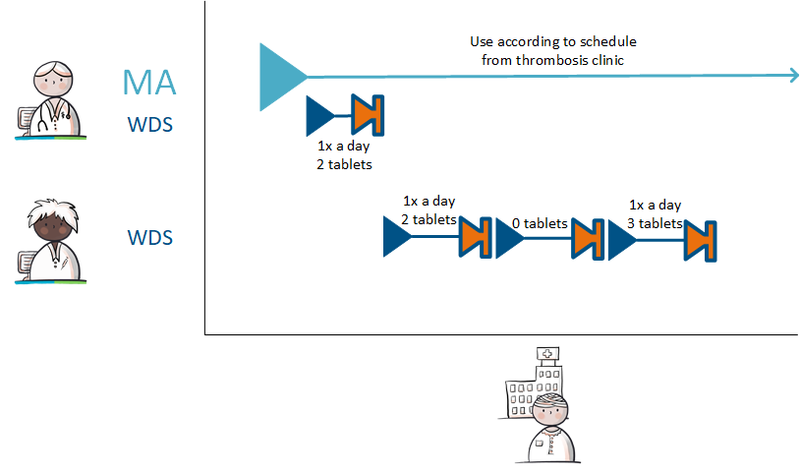
B. (temporarily) halting the anticoagulation medication
Alternatively, the ‘trombosearts’ may choose to (temporarily) halt the anticoagulation medication. In this case, the ‘trombosearts’ creates a stop-MA. This implies that the underlying WDS and the related TA are stopped as well. If the MA should be restarted or adjusted after a certain period, this is done by the initial prescriber of the anticoagulation medication. In general, the thrombosis service may not be involved any more (temporarily); however, if the thrombosis service is still involved, the ‘trombosearts’ may send a VMA to the initial prescriber who can adopt this proposal in an MA.
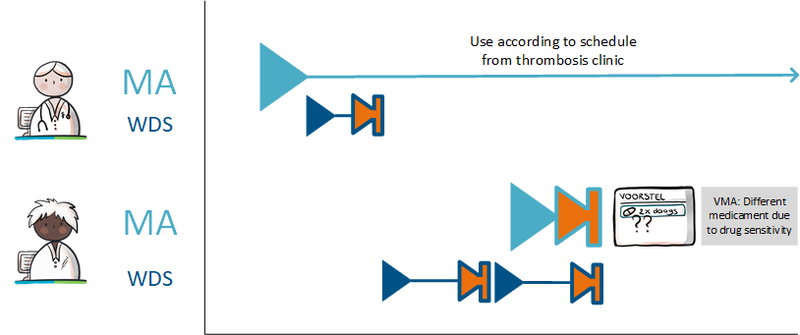
Additional information
Additional information is an extra explanation about the medication use for the (professional) administrator and/or the patient. An example is the situation when fentanyl patch therapy is stopped; additional information is that the patch has to be removed. Another example is the dosing of half a tablet; the additional information contains the instruction what should be done with the other half. This information can be entered as free text, as a comment in the MA, TA and/or WDS.
See use case 4.1.8 Explanation in medicatieafspraak with deliberately chosen special characteristic for more information.
Medication administration deviates from administration list
It may happen that the administration deviates from the instructions as entered by the prescriber and pharmacist in the MA or TA. This may be a deviation in administration time, administered amount, administration route, administration speed, administered product or no administration. A (professional) administrator can deviate from the rules, in consultation with the prescriber, for valid reasons, and under the condition of safe medical practice and safe working practice. The reason for deviation can be entered in the MTD.
Medication administration without medication agreement and administration agreement
In the case of an emergency, the MA and TA may be missing and, therefore, the (professional) administrator has to administer medication on the basis of an MA which is communicated verbally/by telephone. This medication is not incorporated in the administration list. In this situation, the (professional) administrator records the MTD in the information system of the administrator (including ECD, TDR). This is the first building block in a new pharmaceutical treatment; therefore, the administrator creates an MBH.
Medication administration of self-care medication
According to the safety principles in the medication chain, the administrator is not concerned with the administration of self-care medication, except for prescribed selfcare medication (a prescriber has created an MA). In that case, the self-care medication is listed on the administration list as GDS medication or non-GDS medication (as needed).
Correction/cancellation of an administration
This concerns correcting or cancelling an MTD, because an administrator has made a mistake in the registration and no medication has been administered. If the MTD has not been exchanged with other health professionals, the administrator can adjust the MTD by himself, or remove (cancel) the registration from his own information system. If the MTD has been exchanged with other health professionals, the administrator cancels this erroneous MTD and creates a new MTD under the same MBH with the correct information. The administrator (actively) makes the corrected MTD and the new MTD available.
Medication administration on hold
It may happen that the medication administration was unsuccessful, but that is still possible to administer the medication on a later moment, in consultation with a prescriber or pharmacist. If the medication administration is in keeping with the agreements on flexible administration times, the administrator is allowed to deviate from the (guide) administration time.
An example of suspending the medication administration:
- Medication administration is unsuccessful, but the medication can be administered on a later (or, in some cases, earlier) moment of the day. In this situation, the medication administration is suspended, and there is a deviation from the administration list.
Medication administration by a prescriber
A prescriber, similarly to a (professional) administrator, is able to administer medication to a patient, and record this procedure in an MTD.
Multiple administration organizations
Multiple administration organizations may be involved in a patient’s medication administration. For example, medication may be administered by another organization during the evening/night than during the day. If multiple administration organizations are involved in providing patient care, these health professionals can consult each other’s MTDs to track the process of medication administration. The acts of one organization can affect the process of another organization (for example, has medication been administered, have there been deviations from the administration list, etc.).
Feedback to patient through a medication adherence app
In this example the patient uses an app on his mobile phone to help manage his medication. The patient or the pharmacist has created an administration schedule with reminder times (where possible using the app to obtain the toedieningsafspraken from the medicatietoediening). When it is time to take his medication, the patient receives a reminder signal from the app. The patient registers the medication administration and thus indicates whether he has taken the medicine or not (for instance because he forgot to bring it along with him). If the app is linked, for example, to the patient’s PGO (personal health file), medication adherence is tracked automatically.
Use cases, Medicatiegebruik
Self-care product
A patient has acquired Ibuprofen from the drugstore and takes 1200 mg every day. The patient enters this product as gebruik, with such data as starting dates and dosage, and possibly the reason for medicatiegebruik.
Medication from abroad
A patient has been prescribed medication on holiday and is taking this. The patient enters the medication as gebruik, with data such as start date and dosage, and possibly the reason for medicatiegebruik. If known, the name of the prescriber is also recorded.
Modification on the patient’s initiative
The patient has been prescribed propranolol. The patient is having severe problems sleeping because of this and has reduced the medication herself. The patient records gebruik with the modified dosage and the start date for this lower dose. The patient also indicates that she initiated this change herself.
In the case where a patient initiates a dose reduction, the patient can keep on using the medication for a longer period than initially agreed because she still has a supply. The patient can indicate this as additional gebruik.
In the case where a patients initiates a dosage increase, the patient’s supply may run out sooner. The patient may then have to return to the physician earlier than expected.
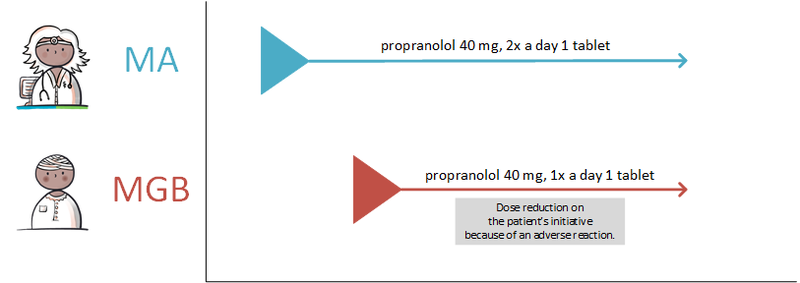
Discontinuation of medication on the patient’s initiative
Since he started using propranolol, the patient suffers from insomnia. He decides to stop without immediately informing the prescribing physician. The patient himself records that he has discontinued medication, indicating the date and giving insomnia as the reason for discontinuing.
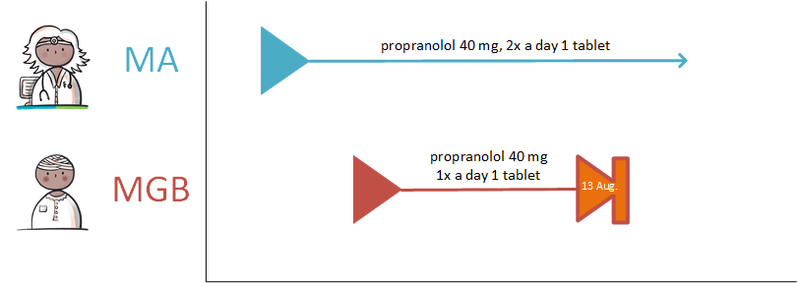
No more supply
A patient has received medication ‘as needed’ for his skin problems. The medication is still active in the medication profile, but is no longer available at the pharmacist. The patient indicates that the medication has been discontinued because it is no longer available, possibly with the addition that the symptoms have disappeared.
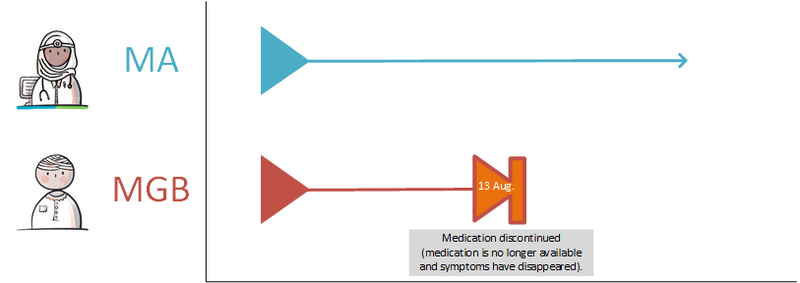
The patient may also make a proposed verstrekkingsverzoek to replenish his supply In this case, the reply proposed verstrekkingsverzoek comes back to the patient (and if approved, a verstrekkingsverzoek is also sent from the prescriber to the pharmacist).
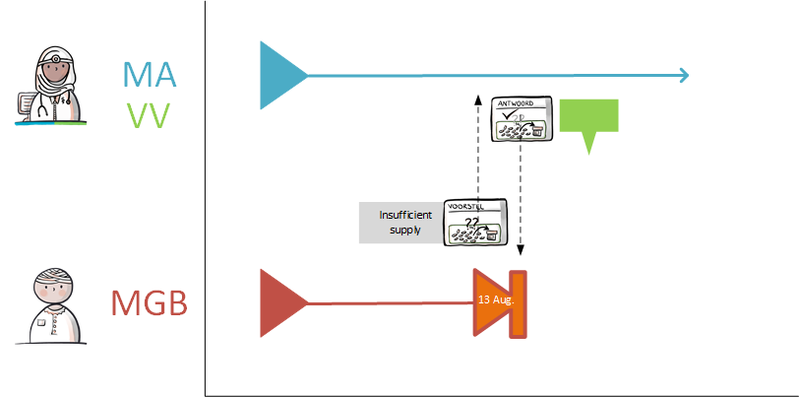
Registrations of abnormal medicatiegebruik by patient due to adverse drug reactions
Since he started taking propranolol, a patient has been suffering from insomnia. The patient records this side effect in the explanatory notes to the medicatiegebruik. When the patient changes the medicatiegebruik of this medication from what was previously agreed, for example when he takes less tablets, he can give the side effect as a reason for this change. When his symptoms diminish or cease, the patient records this in the explanation for medicatiegebruik.
It is possible that the patient is suffering from side effects without exactly knowing which medicinal products cause them. There is no provision within medication process to record this (yet).
Register medicatiegebruik based on provision
This applies in the transition period from the old medication process standard 6.12 to this medication process information standard.
Only verstrekking (dispensing) is available digitally (according to medication process version 6.12). However, the user's information system offers the possibility to record all medicatiegebruik. In that case, the medicatiegebruik can be recorded with reference to the ID of the version 6.12 verstrekking.
Medication overview and inference rules
This chapter shows a sample medication overview and also specifies inference rules for 'The most current relevant building block', Verification for each medicamenteuze behandeling, etc.
Introduction
The term 'current medication overview' has been replaced by the term 'basisset medicatiegegevens’ (basic set of medication data): the medication overview in combination with additional information that is required (at least) to be able to prescribe, change, stop or safely provide medication in a safe and responsible manner in the Transfer of Medication Data in the Chain (Leidraad Overdracht van Medicatiegegevens in de keten, final draft 2017).
This page contains an example of a medication overview. This example was developed in the Medication Process Information Standard program and serves as an example of how data can be displayed in an application. The data numbered in the pictures below are normative, they are mandatory to show. The other (unnumbered) data in the example is optional and may be added as normative in the future. The layout of the overview in a information system can differ per target group and per device. For pharmacists, for example, only toedieningsafspraken are initially visible, after which it is possible to click further to get to the corresponding medicatieafspraak and the medicatiegebruik. If only medicatiegebruik or a medicatieafspraak is known, this will of course be shown immediately. The basic principle is that the overview shows all medication of the patient, not just the medication that is registered in the patient's own information system.
The overview is described generically and therefore the same for all suppliers. The requirements of the end users are included on this page and no statements are made about the technical realization or implementation. The existing KNMP specification “User requirements specification Medication overview 2.0”, final concept “Guidance Transfer of Medication data in the chain (Leidraad OVerdracht van Medicatiegegevens in de keten)” and the new insights from the Medication process program have been incorporated in this.
Principles for the overview are:
- Health professionals want to see a distinction between the therapeutic building blocks on a medication overview:
- Medicatieafspraak
- Toedieningsafpsraak
- Medicatiegebruik
- Logistical building blocks (verstrekkingsverzoek, dispensing) and the medicatietoedieningbuilding block are not relevant for the medication overview.
Functional specification
In the figures below the parts with normative data are included. The other parts are not yet normative. The numbered elements in the table are normative, other elements are optional for the time being.
Heading and General
Filter: N/A
Sort: N/A
Medication Overview Date: This is the date that the last change to a medicatieafspraak, toedieningsafspraak or medicatiegebruik was recorded within the information system providing the medication overview.
NOTE: In the viewer, all dates are shown with the month in characters, so that there is no confusion between day-month and month-day (American format).
| No | Header | Dataset | Explanation |
|---|---|---|---|
| 1 | Name | Patient – Name data (Initials, Surname, PartnerSurname), Date of Birth, Gender | |
| 2 | Telephone | Patient – Contact data – Telephone numbers | Primary telephone number of patient |
| 3 | BSN | Patient – Patient identification number | Always BSN |
| 4 | Adress | Patient – Address data | |
| 5 | Healthcare Provider Name | Healthcare Provider – Organization name | Healthcare Provider who has compiled the medication overview |
| 6 | Healthcare Provider Adress | Healthcare Provider – Address – Address data | Healthcare Provider who has compiled the medication overview |
| 7 | Healthcare Provider Telephone | Healthcare Provider – TelephoneMail – Contact data – Telephone numbers | Healthcare Provider who has compiled the medication overview |
| 8 | Healthcare Provider Email | Healthcare Provider – TelephoneMail – Contact data – Email addresses | Healthcare Provider who has compiled the medication overview |
| 9 | Date of Medication Overview | Document data – Document date | |
| Height | Body height – HeightValue, HeightDateTime | ||
| Weight | Bodyweight – WeightValue, WeightDateTime | ||
| Checked by Health professional | Document data – Verification by health professional | ||
| Verified with Patient/Informal Caregiver | Document data – Verification by patient |
Elements 5 through 8 are not shown if the patient is the creator of the medication summary.
Contraindications and hypersensitivities (CiO)
Active contraindications and hypersensitivities (intolerances, allergies/adverse reactions) (in Dutch: 'contraindicaties en overgevoeligheden' - CiO).
Filter: end date is not filled, is in the future or was less than a year ago when compiling the overview.
Sort: most recent effective date at the top.
This part will be elaborated at a later time and is not yet normative.
Current medication
Filter: all current medication, i.e. all medicatieafspraken and toedieningsafspraken that apply at the time the overview is compiled and the associated medicatiegebruik per source. The temporarily interrupted medication is also shown in this category. When only medicatiegebruik is known, it is shown if it is not older than 13 months. When medicatiegebruik is recorded by both a health professional and a patient, the last registered medicatiegebruik is shown for both.
Sorting: by medicamenteuze behandeling (MBH): medicatieafspraak, toedieningsafspraak, medicatiegebruik (recorded by patient, care provider). Medicamenteuze behandelingen are sorted in descending order by startdate medicatieafspraak, toedieningsafspraak, medicatiegebruik.
| No | Header | Dataset | ||
|---|---|---|---|---|
| 1 | Type | Medicatieafspraak | Toedieningsafspraak | Medicatiegebruik |
| 2 | Medicinal product | Agreed medicinal product- | Medicinal product belonging to Toedieningsafspraak- | Device- |
| Product – ProductCode (if absent: ProductSpecification, ProductName) | ||||
| 3 | Start date | Start date | ||
| 4 | End date/duration | End date (if not available: Duration) | ||
| 5 | Dosage | Instructions for medicatiegebruik – Description | ||
| 6 | Route of toediening | Instructions for medicatiegebruik – Route of toediening | ||
| 7 | Reason | Reason for prescribing &
if applicable reason for discontinuing/suspending medication |
Reason for discontinuing/suspending medication | Reason for discontinuing/suspending medication |
| 8 | Explanation | Explanation & additional information | Explanation | |
| 9 | Source | Prescriber- | Provider- | Author- |
| Health professional – Name healthcare provider, specialty | ||||
| n/a | n/a | or “Patient” | ||
Future medication
Filter: all medication that will become actual in the next 3 months. This includes intended medicatiegebruik. For a description of the mapping, the same applies as with the current medication, see Current medication.
Recently discontinued medication
Filter: any medication that has ended or stopped in the past 2 months.
For a description of the mapping, the same applies as with the current medication, see Current medication.
Additional lab values
Most recent lab values and abnormal renal function values.
This part will be elaborated at a later time and is not yet normative.
Remarks
For example, relevant (limited) health skills (competences: literacy, calculation and digital skills) that can have an impact on medicatiegebruik / treatment.
This part will be elaborated at a later time and is not yet normative.
Building block instantiations
This section describes which instantiations of the building blocks belong to the medication overview. This concerns 'own' and 'other people's' building blocks (section 3.1) and then only the latest, relevant instantiations of this.
Own and other people
Both the own and a copy of (known to the sender) building blocks from other sources are included in the medication overview. This so that the medication overview is as complete as possible for the recipient. This ensures that recipients can start using it immediately and that they are not dependent on other sources. The technical representation of someone else's building block may differ from that of the real source. It is important that the recipient is aware of this. The original OID of someone else's building block should simply be given. The recipient can then choose to also collect the building block from its source in its original form.
The medicatieafspraak, toedieningsafspraak and medicatiegebruik building blocks have been extended with a feature that indicates whether:
- there is an 'own' building block or
- that of "someone else" (an accent building block: MA’, TA’, MGB’)
This attribute only applies in the medication overview transaction, because that is the only transaction where other people's building blocks may be delivered.
The 'building block of someone else' attribute is on two levels:
- The template ID of an MA’, TA’, MGB’ differs from an MA, TA and MGB.
- In addition, the element copy indicator is always sent with value true in MA’, TA’, MGB’.
For the medication overview, it is permitted to offer the medicatieafspraken, toedieningsafspraken and medicatiegebruik via a information system-generated MGB or MGB '(Healthcare provider is author MGB). This must have a reference to an MA, TA or MGB.
Medication overview and derivation rules
An overview of medication that the patient is using (or should be using) can be put together in a information system in two ways:
1) displaying a current medication overview (or overviews) obtained from another source (or sources)
2) showing coherent medication building blocks obtained from one's own information system and from other sources.
In both cases, this overview of medication consists of the information as included in the building blocks medicatieafspraak, toedieningsafspraak and medicatiegebruik.
Re 1) When this document refers to a current medication overview, this means a coherent overview in which current medicatieafspraken, toedieningsafspraken and medicatiegebruik are included. This current medication overview is built up by the source on the basis of the data known at that time at the source. This overview can be exchanged with the transaction group Medicatieoverzicht (Medication overview) (PULL or PUSH).
The data and building blocks that are known at the source, but originating from another source, are supplied in the transaction with the 'accent' indicator so that they are recognizable as building blocks from someone else (MA accent, TA accent, MGB accent).
Re 2) It is also possible for a information system to compile an overview by combining own and other people's separate individual medication building blocks. The transaction group Medicatiegegevens (medication data) (PULL) is used to request individual building blocks.
With the transaction group Medicatiegegevens (medication data) (PUSH), separate building blocks can also be sent directly to another health professional or the patient (so without requests). This happens, for example, at discharge or at the request of a co-practitioner who has the patient in front of him (e.g. patient appears to a GP or pharmacist outside the region, where this GP or pharmacist requests the data by telephone from the patient's own GP and receives it digitally).
Both Medicatiegegevens (medication data) PUSH and PULL should not provide data obtained from other sources.
Last relevant
Only the last relevant building blocks (per medicamenteuze behandeling) are part of the medication overview. This section describes what exactly those last relevant building blocks are for a medication overview. Firstly for prescription medication (section 3.2.1), then for "over the counter" medication (section 3.2.2). The following section 3.2.3 further discusses the special situation that, in addition to a current, a future medicatieafspraak within the same medicamenteuze behandeling is valid. Finally, the last paragraph summarizes the rules to be applied.
Note: The (technical) stop-medicatieafspraken are not shown in the examples, but are implicitly assumed.
Prescription medication
Most simple "happy flow"
In a medicamenteuze behandeling, the simplest "happy flow" results in a sequence of:
- Medicatieafspraak – Toedieningsafspraak – Medicatiegebruik.
Usually, medicamenteuze behandeling (drug treatment) starts with a medicatieafspraak. A toedieningsafspraak concretely completes the medicatieafspraak. medicatiegebruik says something about the actual medication use of the patient in this medicamenteuze behandeling. All three building blocks are relevant in such a case and are part of the delivery of a medication overview, as indicated in green below.
- Medicatieafspraak – Toedieningsafspraak – Medicatiegebruik.
A toedieningsafspraak refers to the medicatieafspraak he enters. If no referral is available in the toedieningsafspraak, the appointment date (administration appointment) must be later than that of the medicatieafspraak to be relevant to delivery.
Medicatiegebruik can refer to a medicatieafspraak or toedieningsafspraak. If no referral is available in the medicatiegebruik, the registration date (medicatiegebruik) must be later than that of the medicatieafspraak to be relevant for delivery.
New medicatieafspraak in medicamenteuze behandeling
If you create a new medicatieafspraak in an existing medicamenteuze behandeling (drug treatment), the currently existing medicatieafspraken, toedieningsafspraken and records of medicatiegebruik are no longer relevant for the medication overview. The idea is that all older building blocks than the current medicatieafspraak are by definition "overruled" by this new medicatieafspraak and consequently no longer relevant for the medication overview.
- Medicatieafspraak – Toedieningsafspraak – Medicatiegebruik - stop-Medicatieafspraak – Medicatieafspraak – stop-Toedieningsafspraak
If a toedieningsafspraak and possibly also medicatiegebruik are created and available following such a medicatieafspraak medication appointment, these will be included in the medication overview.
- Medicatieafspraak – Toedieningsafspraak – Medicatiegebruik – stop-Medicatieafspraak – Medicatieafspraak – stop-Toedieningsafspraak - Toedieningsafspraak
- Medicatieafspraak – Toedieningsafspraak – Medicatiegebruik – stop-Medicatieafspraak - Medicatieafspraak - stop-Toedieningsafspraak – Toedieningsafspraak – Medicatiegebruik
The new MA can also be a stop-MA, if the patient has to stop medication permanently. This stop-MA remains relevant for the medication overview for 2 months.
- Medicatieafspraak – Toedieningsafspraak – Medicatiegebruik – stop-Medicatieafspraak - stop-Toedieningsafspraak
Medicatiegebruik earlier than toedieningsafspraak
It is possible that you have registered medicatiegebruik before the toedieningsafspraak has been made. Here too, the idea is that the newer toedieningsafspraak "overrules" the older registration of toedieningsafspraak and medicatiegebruik. The older medicatiegebruik is therefore no longer relevant for the medication overview.
- Medicatieafspraak – Medicatiegebruik
- Medicatieafspraak – Medicatiegebruik – Toedieningsafspraak
Of course, a new record of medicatiegebruik can then be made, which is also relevant for the medication overview.
- Medicatieafspraak – Medicatiegebruik – Toedieningsafspraak - Medicatiegebruik
New toedieningsafspraak
Sometimes you make a new toedieningsafspraak under an existing medicatieafspraak. For example, because another product must be provided (as a result of preference policy or stock). In that case, too, such a new toedieningsafspraak may overrule older medicatieafspraken and medicatiegebruik.
- Medicatieafspraak – Toedieningsafspraak – Medicatiegebruik- stop-Toedieningsafspraak - Toedieningsafspraak
Of course you can then record medicatiegebruik, which is also relevant for the medication overview.
- Medicatieafspraak – Toedieningsafspraak – Medicatiegebruik - stop-Toedieningsafspraak – Toedieningsafspraak - Medicatiegebruik
Over the counter
Sometimes a patient uses medication for which there is no prescription. This applies, for example, to painkillers such as paracetamol or ibuprofen, which are available without a prescription. We call this “over the counter” medication. Such use of "over the counter" medication can be recorded with the medicatiegebruik building block. This is also relevant for the medication overview.
- Medicatiegebruik
A new record of medicatiegebruik by the same type of author (type of author is patient or health professional) "overrules" any older records of medicatiegebruik. Elaborated below, in order of registration date:
- Medicatiegebruik - Medicatiegebruik
When a prescriber decides to formalize this medicamenteuze behandeling with a medicatieafspraak the rules as described above apply. Elaborated below, in order of appointment date (medicatieafspraak or toedieningsafspraak) / registration date (medicatiegebruik):
- Medicatiegebruik – Medicatiegebruik – Medicatieafspraak
- Medicatiegebruik – Medicatiegebruik – Medicatieafspraak - Medicatiegebruik
- Medicatiegebruik – Medicatiegebruik – Medicatieafspraak – Toedieningsafspraak - Medicatiegebruik
- Medicatiegebruik – Medicatiegebruik – Medicatieafspraak – Medicatiegebruik - Toedieningsafspraak - Medicatiegebruik
- Medicatiegebruik – Medicatiegebruik – Medicatieafspraak - Medicatiegebruik - Medicatiegebruik
Future medicatieafspraken and toedieningsafspraken
A medicamenteuze behandeling may include more than one topical medicatieafspraak and toedieningsafspraak. Namely one for the current situation and one or more for the future situation. This section describes an example with one current and one future. For clarity, the future building blocks have a “T-” in the examples. Both current and future building blocks are relevant for the medication overview. The building blocks for toedieningsafspraak and medicatiegebruik must have adequate references to either the current or future appointments. Below are three examples, in order of appointment date / registration date (if these are equal, then sorted by period of use):
- Medicatieafspraak – T Medicatieafspraak
- Medicatieafspraak – Toedieningsafspraak – T Medicatieafspraak – T Toedieningsafspraak
- Medicatieafspraak – Toedieningsafspraak – Medicatiegebruik – T Medicatieafspraak – T Toedieningsafspraak – T Medicatiegebruik
Suppose that the toedieningsafspraak changes because the provider provides a different strength (2x 500mg tablets instead of 1x 1000mg tablets) and the patient still has enough stock for two weeks based on the first toedieningsafspraak:
- Medicatieafspraak – Toedieningsafspraak – Medicatiegebruik – T Toedieningsafspraak – T Medicatiegebruik
New medicatieafspraak
If you make a new medicatieafspraak in this situation, you always make a (technical) stop-medicatieafspraak. This stop-medicatieafspraak has two manifestations:
1) with reference to a specific medicatieafspraak that you stop
2) without a referral, and with that you stop the entire medicamenteuze behandeling (drug treatment)
Ad 1) Stop medicatieafspraak for one current medicatieafspraak. With this stop medicatieafspraak you stop one specific medicatieafspraak. You also make a new one for that medicatieafspraak. Suppose you only want to change the current medicatieafspraak, then this applies to the examples above, in order of appointment date:
- Medicatieafspraak – T Medicatieafspraak - stop-Medicatieafspraak – Medicatieafspraak
- Medicatieafspraak – Toedieningsafspraak – T Medicatieafspraak – T Toedieningsafspraak - stop-Medicatieafspraak – Medicatieafspraak
- Medicatieafspraak – Toedieningsafspraak – Medicatiegebruik – T Medicatieafspraak – T Toedieningsafspraak – T Medicatiegebruik - stop-Medicatieafspraak – Medicatieafspraak
Suppose you only want to change the future medicatieafspraak, then this applies to the examples above, in order of appointment date:
- Medicatieafspraak – T Medicatieafspraak - stop- T Medicatieafspraak – T Medicatieafspraak
- Medicatieafspraak – Toedieningsafspraak – T Medicatieafspraak – T Toedieningsafspraak - stop- T Medicatieafspraak – T Medicatieafspraak
- Medicatieafspraak – Toedieningsafspraak – Medicatiegebruik – T Medicatieafspraak – T Toedieningsafspraak – T Medicatiegebruik - stop- T Medicatieafspraak – T Medicatieafspraak
Ad 2) Stop medicatieafspraak for the entire medicamenteuze behandeling. With this stop medicatieafspraak you stop all existing (current and future) medicatieafspraken in the medicamenteuze behandeling. If you now want to make a medicatieafspraak for the future in addition to a current one, you must also make it again. Elaborated below for the same three examples as above, in order of appointment date.
- Medicatieafspraak – T-Medicatieafspraak – stop-Medicatieafspraak (zonder referentie) - Medicatieafspraak
- Medicatieafspraak – Toedieningsafspraak – T-Medicatieafspraak – T-Toedieningsafspraak - stop-Medicatieafspraak (zonder referentie) – Medicatieafspraak – T Medicatieafspraak
- Medicatieafspraak – Toedieningsafspraak – Medicatiegebruik – T-Medicatieafspraak – T-Toedieningsafspraak – T Medicatiegebruik - stop-Medicatieafspraak (zonder referentie) – stop-Toedieningsafspraak -Medicatieafspraak – stop- T Toedieningsafspraak - T Medicatieafspraak
New toedieningsafspraak
However, you can also make a new toedieningsafspraak for the current medicatieafspraak only. This new toedieningsafspraak must then have a reference to that current medicatieafspraak. Elaborated below for three examples, in order of appointment date.
- Medicatieafspraak – T Medicatieafspraak – Toedieningsafspraak
- Medicatieafspraak – Toedieningsafspraak– T-Medicatieafspraak – T Toedieningsafspraak - stop-Toedieningsafspraak – Toedieningsafspraak
- Medicatieafspraak – Toedieningsafspraak – Medicatiegebruik – T Medicatieafspraak – T-Toedieningsafspraak – T Medicatiegebruik – - stop-Toedieningsafspraak – Toedieningsafspraak
A new toedieningsafspraak with a reference to the future medicatieafspraak looks like this.
- Medicatieafspraak – T Medicatieafspraak – Toedieningsafspraak – T-Toedieningsafspraak
- Medicatieafspraak –Toedieningsafspraak – T-Medicatieafspraak – T Toedieningsafspraak – stop-T Toedieningsafspraak - T Toedieningsafspraak
- Medicatieafspraak –Toedieningsafspraak – Medicatiegebruik – T Medicatieafspraak – T Toedieningsafspraak – T Medicatiegebruik - stop- T Toedieningsafspraak – T-Toedieningsafspraak
Rules of overruling listed
Based on the above description / examples, the following general rules for overruling can be derived. This concerns the rules for determining whether a building block is relevant for delivery in the medication overview. Within a medicamenteuze behandeling:
- A new medicatieafspraak overrules all older building blocks (medicatieafspraak, toedieningsafspraak, medicatiegebruik) belonging to the medicatieafspraak /medicatieafspraken that this medicatieafspraak has stopped.
- A new toedieningsafspraak overrules all older building blocks of the type of toedieningsafspraak or medicatiegebruik that belong to the same medicatieafspraak as the new toedieningsafspraak.
- In principle, a new record of medicatiegebruik overrules (see exception below) older records of medicatiegebruik that belong to the same medicatieafspraak as the new medicatiegebruik.
- Exception to this rule: medicatiegebruik with the author as a patient DOES NOT overrule an older record of medicatiegebruik with the care provider as author and vice versa. Both are therefore relevant to the medication overview.
The latter exception applied to a number of situations:
Deze laatste uitzondering toegepast op een aantal situaties:
- Medicatieafspraak – Toedieningsafspraak – Medicatiegebruik-ZVL – Medicatiegebruik-PAT
- Medicatieafspraak – Toedieningsafspraak – Medicatiegebruik-PAT – Medicatiegebruik-ZVL
- Medicatieafspraak – Toedieningsafspraak – Medicatiegebruik-PAT – Medicatiegebruik-PAT
- Medicatieafspraak – Toedieningsafspraak – Medicatiegebruik-ZVL – Medicatiegebruik-PAT – Medicatiegebruik-PAT
- Medicatieafspraak – Toedieningsafspraak – Medicatiegebruik-ZVL – Medicatiegebruik-PAT – Medicatiegebruik PAT – Medicatiegebruik-ZVL
Verification by medicamenteuze behandeling
A health professional records verification per medicamenteuze behandeling with the medicatiegebruik building block:
- Author = Health professional
- Possible informant: the patient, a care provider or another person
- Possible characteristic "according to agreement": according to the author, is the medicatiegebruik in accordance with the medicatieafspraak or toedieningsafspraak?
- Possible link to medicatieafspraak or toedieningsafspraak:
- Medicatieafspraak or toedieningsafspraak that has been the reference for recording this medicatiegebruik.
- When it is indicated "by appointment", this is the medicatieafspraak or toedieningsafspraak according to which the patient uses.
Such a record of medicatiegebruik means: "I think the patient uses this drug as follows: ... ..".
Known: it is not currently provided in the information standard to indicate that a medicatieafspraak or toedieningsafspraak is correct, regardless of whether the patient uses it as such.
Process of the medication overview exchange
In addition to the content and verification, the process surrounding the medication overview is also important.
Who, when, with what information
The reliability and completeness of a medication overview depends on:
- who composed it,
- at what time and
- with what basic information.
We can make agreements about this, for example:
- only exchange/make available a medication overview once it has some form of reliability
- after an explicit action by a health professional or
- automatically if certain conditions are met.
- fully automatically exchange/make available medication overview without conditions
- make medication overview fully automatically available without the need for a new data type (so when registering one of the other medication data types you are automatically also the source of a medication overview).
Decision
The above questions have not yet been answered. It has been decided that during the Proof-Of-Concept all parties that can provide a medication overview will do the same.
Responsibilities applicant and source
The applicant receives medication overviews from multiple sources. Processing this is the responsibility of the recipient. The topicality of a medication overview is important here.
Action: the transaction Medicatieoverzicht (medication record) must contain the most recent date of the collection of available appointment dates / registration dates of the delivered building block instantiations. The compiler (source) of the medication overview must therefore provide this date. This helps the recipient to better estimate the topicality of the medication overview.
Agreements continued
We continue the analysis with the results of the Proof-Of-Concept to arrive at a good process for the medication overview.
Inference rules
It is important that all parties, physician, pharmacist, nurse as well as patient can infer from the medication building blocks what is intended (i.e. what the current agreements are) and that this should be the same for all information systems. For that reason, information systems must be able to calculate the current situation using the same inference rules.
The current therapeutic situation is calculated on the basis of medicatieafspraken and toedieningsafspraken. Toedieningsafspraken have a relationship with the medicatieafspraak that they were added to. For this reason, toedieningsafspraken can be linked to the medicatieafspraak that they belong to.
Of course it is also useful to have information about medicatiegebruik. However, this does not indicate the aim of the health professional, only what medication the patient has used. For that reason, the rules below do not take ‘medicatiegebruik’ in consideration. However, ‘gebruik’ is part of a medication profile but is shown separately under the medicamenteuze behandeling concerned.
Effective period
The effective period lies between an effective start date and an effective end date.
- The effective period describes the period to which a medicatieafspraak or toedieningsafspraak (ultimately) applies.
The effective period depends on adjustments (modifying/discontinuing/halting/resuming) of medication and by the ‘actual handling of a medicatieafspraak by a toedieningsafspraak. The effective period is not described in the dataset, because it is inferred which is only used to determine the current situation. The effective start date, effective end date and effective period are not data to be shown to the end user. Medication for an indefinite period has an effective end date that is in the future, but at a time that cannot be distracted (yet). It is only known that it is 'somewhere' in the future.
Actual and current medication
- Actual (medicatieafspraken en toedieningsafspraken): the agreements of which the effective end date lies in the future.
- Current (medicatieafspraken en toedieningsafspraken) agreements: the agreements for which the present date lies between their effective start date and the effective end date.
- Actual medication: the collection of actual (current and future) medication and toedieningsafspraaks as well as current medicatiegebruik. It should be taken into account that it is never possible to say with certainty to what extent the patient complies with the agreements and/or reports actual gebruik.
Details
Approach
Using a number of different situations the inference rules are described for arriving at the effective therapeutic period in a medication profile:
- 1) Starting medication
- 2) Creating a toedieningsafspraak
- 3) Changing medication
- 4) Discontinuing medication
- 5) Temporarily halting and resuming medication
The inference rules are described below. The number for each inference rule indicates the order in which the rules must be executed.
1) Starting medication
Medication is started by creating a new medicatieafspraak. The medicatieafspraak has:
- An agreement date - the date on which the agreement was made with the patient.
- A period of use - this period is consists of:
- A start date - the start date of the medicatieafspraak may be in the future;
- The duration of medicatiegebruik;
- The end date – the date until which the medicatieafspraak is valid.
1a: The effective period of a new medicatieafspraak is equal to its period of medication use.
2) Creating a toedieningsafspraak
A toedieningsafspraak specifically fulfils a medicatieafspraak. From a patient perspective, the toedieningsafspraak, in a way, replaces a medicatieafspraak, as the toedieningsafspraak is a more specific indication of what the patient should be using.
Inference rule 1 is extended:
1b: The effective period of a new toedieningsafspraak is equal to its period of medication use
Inference rule 2 is applicable when a medicatieafspraak has underlying toedieningsafspraken (see inference rule 2b for further explanation):
2a: The effective period of a medicatieafspraak is equal to the effective period of the toedieningsafspraak.
3) Changing medication
- a) By means of a medicatieafspraak
The prescriber changes the medication (or the medicamenteuze behandeling) by creating a new medicatieafspraak (and discontinuing the existing one) within an existing medicamenteuze behandeling. Because the start date of a medicatieafspraak may lie in the future, several medicatieafspraken may be actual at the same time. For example, when a medicatieafspraak applies ‘for an indefinite period’ (ma1) and it is agreed to change the dosage in two weeks (ma2), the first medicatieafspraak (ma1) remains valid for two weeks, after which the second medicatieafspraak (ma2) becomes effective. The previous inference rules do not change because of this.
- b) By means of a toedieningsafspraak
An toedieningsafspraak specifically fulfils a medicatieafspraak. This toedieningsafspraak may be modified. For example, when administration schedules are changed (when GDS is initiated), or when a commercial product is changed (for example, as a result of a preference policy). Several successive toedieningsafspraken can therefore be created under a single medicatieafspraak. Hence, rule 2 is expanded so that the effective period of the medicatieafspraak is determined by the entire series of underlying toedieningsafspraken.
2b: When several toedieningsafspraken are made under a single medicatieafspraak, the effective start date of the medicatieafspraak is then equal to the earliest start date of the underlying toedieningsafspraak and the end date of the last toedieningsafspraak.
Parallel toedieningsafspraken may exist under a single medicatieafspraak of which the agreement date and the start date are the same. Both are then valid at the same time and this does not change rule 2b.
The diagram below includes a simplified example of an actual profile composed of different building blocks. The patient, verification, CiO and lab data have been excluded from this overview. Only limited data has been included, mainly to demonstrate the way in which the effective period works. Lines starting with ‘-’ are detail lines which can be hidden in the medication profile, if required. The first line shows the effective period for the underlying MAs and TAs. The ‘medicatiegebruik” is also displayed, but this does not influence the calculation of the effective therapeutic period on the first line.
4) Discontinuing medication
Discontinuing medication (i.e medicamenteuze behandeling) is done by creating a new medicatieafspraak in which stoptype ‘definite’ is indicated. The start date of this medicatieafspraak is the date on which the original medicatieafspraak started. The end date is the date on which the medication is discontinued. The effective period of the original medicatieafspraak is so replaced by the effective period of this new stop-MA.
1c: When a medicatieafspraak is discontinued, the effective period of the medicatieafspraak is then the effective period of the stop-MA.
An stop-toedieningsafspraak fulfils the stop-medicatieafspraak. In this way, for example, it is possible to indicate that the discontinuation starts when the next GDS roll is dispensed.
5) Temporarily halting and resuming medication
Temporarily halting medication is carried out in the same way as discontinuing medication. A stop-MA is created (stoptype ‘temporary’) with the same start date as that of the original medicatieafspraak. The end date of the stop-MA is the timing of the temporary halt, the stop type is ‘temporary’. Resuming medication is done by creating a new medicatieafspraak with the old (or adjusted) dosage with the date of resuming as the start date. A meaningful reason (example: resume previous policy) may serve to clarify matters. If the medication is not resumed, ‘discontinue medication’ (stoptype ‘definite’) can be executed in order to permanently end the medicamenteuze behandeling, which means the medication is no longer valid. Medication that is halted remains valid, and so remains visible in the medication profile.
1d: When medication is temporarily halted, the effective period is not affected. The effective period is determined by the effective period of the original medicatieafspraak.
These inference rules are the basis for determining the current agreements. It is possible to conceive exceptional situations relating to a partial availability of medication data. These situations have not been elaborated. All inference rules are included and implemented in the open source medication viewer.
Data that need not be shown
Various data are important for the correct processing of information, but are not relevant to the end user:
- An application does not have to show the (technical) identification of a medicamenteuze behandeling (MBH) (nor that of the building blocks themselves), but the elaboration thereof: namely, building blocks shown in coherence.
- The copy indicator with the medicatieafspraak (MA), toedieningsafspraak (TA) and medicatiegebruik (MGB) when consulting the medication overview. Whether a building block is a copy is not that important to a user and can be deduced by other data from that building block (such as the author of the building block). So it is not so important that a user can see that this has been delivered 'as a copy' and is probably only confusing. However, this information is important for suppliers, for example because they sometimes want to perform calculations with the data and it is then safer to collect the data from the real source. It is therefore obvious to keep track of whether you have received data from the real source in your information system.
- An application does not have to show the stop type (temporary / definite) of a stop appointment, but the elaboration thereof: namely the processing of the meaning, so that an entire MBH is shown as stopped medication or as interrupted medication (remains clear under current medication) or as a change in the case of a 'technical stop appointment'.
Administration list and inference rules
This chapter shows a possible presentation/view of the administration list and, additionally, specifies the inference rules to identify the relevant medication building blocks within a pharmaceutical treatment, for the generation of an administration list.
Introduction
This paragraph contains an example of an administration list. The administration list shows the most recent medication data at any time of the day, to facilitate adequate medication administration. To ensure safe circumstances in which correct and up-to-date medication data is presented to the (professional) administrator, the administration software must be able to process the medication building blocks and to correlate the medication building blocks, as described in this information standard.
The term ‘administration list’ is defined as a digital list including all medicaments that are prescribed by a prescriber and that have to be administered to a patient/client. This list is generated based on the following medication building blocks: medication agreement (MA), administration agreement (TA), medication dispense (MVE), medication administration (MTD) and variable-dosing regimen (WDS).
The process of (actively) making medication data available to (professional) administrators enables the generation of a total overview of all medication which has to be administered. Importantly, this implies that information regarding discontinuation and change of medication with or without medication dispense is available at any time. Change in medication and variable-dosing regimens, for example as prescribed by the thrombosis service, are immediately available for administrators.
In the figures below, the numbered elements are normative; it is mandatory to show those components. The lay-out of the administration list in an information system may differ depending on the type of users or the type of device; this is dependent on the user requirements.
The administration list in this example is described generally. The chapter entails the general end user requirements for the administration list; this chapter does not comprise details on the technical requirements or implementation.
The project ‘Veilige principes in the medication’ comprises the sectors nursing care, nursing aid and home care. The specifications as described in ‘Veilige principes in de medicatieketen 2016’ and ‘Richtlijn overdracht van medicatiegegevens in de keten herziening 2018/2019’ as well as new insights from the program ‘Medicatieoverdracht’ are incorporated in this chapter.
The basis for the administration list entails:
- Medication building blocks
- Medication agreement (MA)
- Administration agreement (TA)
- Variable-dosing regimen (WDS)
- Medication administration (MTD)
- Medication dispense (MVE)
- All current medication; this comprises all MAs, TAs and WDSs which are available and applicable when the medication is administered. The PeriodOfUse defines whether medication should be considered current medication.
- The PrickPatchLocation is consulted based on the AdministrationDateTime.
- (Professional) administrators can distinguish the types of medication (in GDS package or separately) which should be administered to the patient by the data element DistributionForm (medication building block MVE) and the data element AsNeeded/Condition in the dosage (medication building blocks MA, TA or WDS). The different types of medication may be presented according to the following categories:
- GDS medication (including discontinued medication in order to show a notification/warning)
- Non-GDS medication
- Non-GDS medication as needed
- The remaining building blocks (dispense request (VV), medication use (MGB) and medication consumption (MVB)) are not relevant for the administration list.
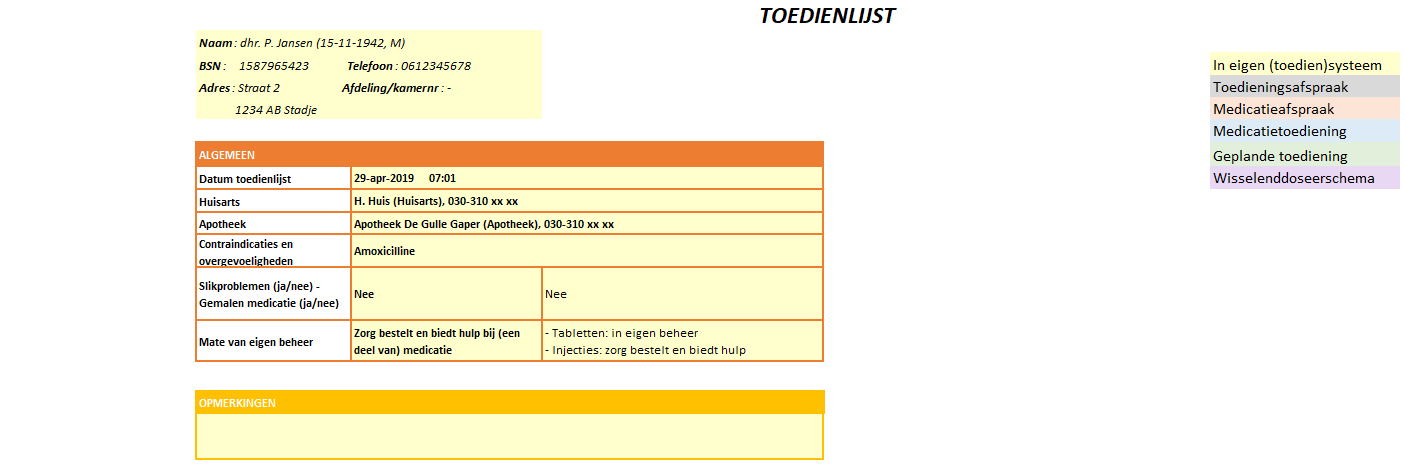
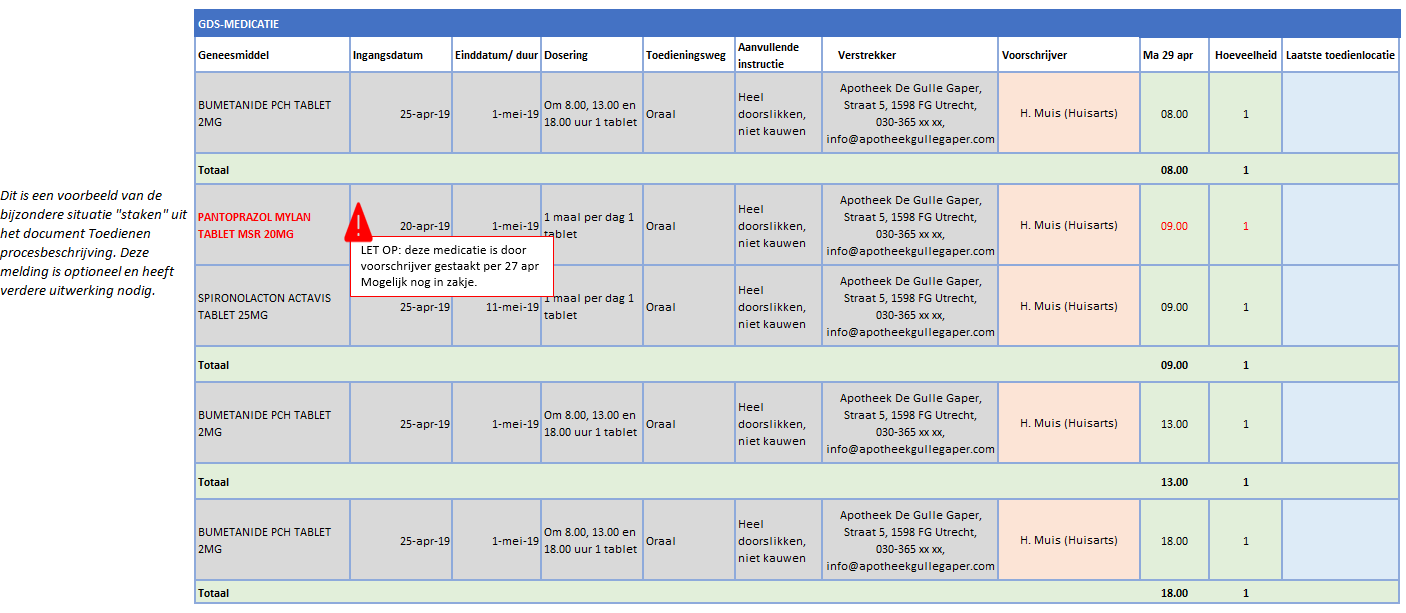
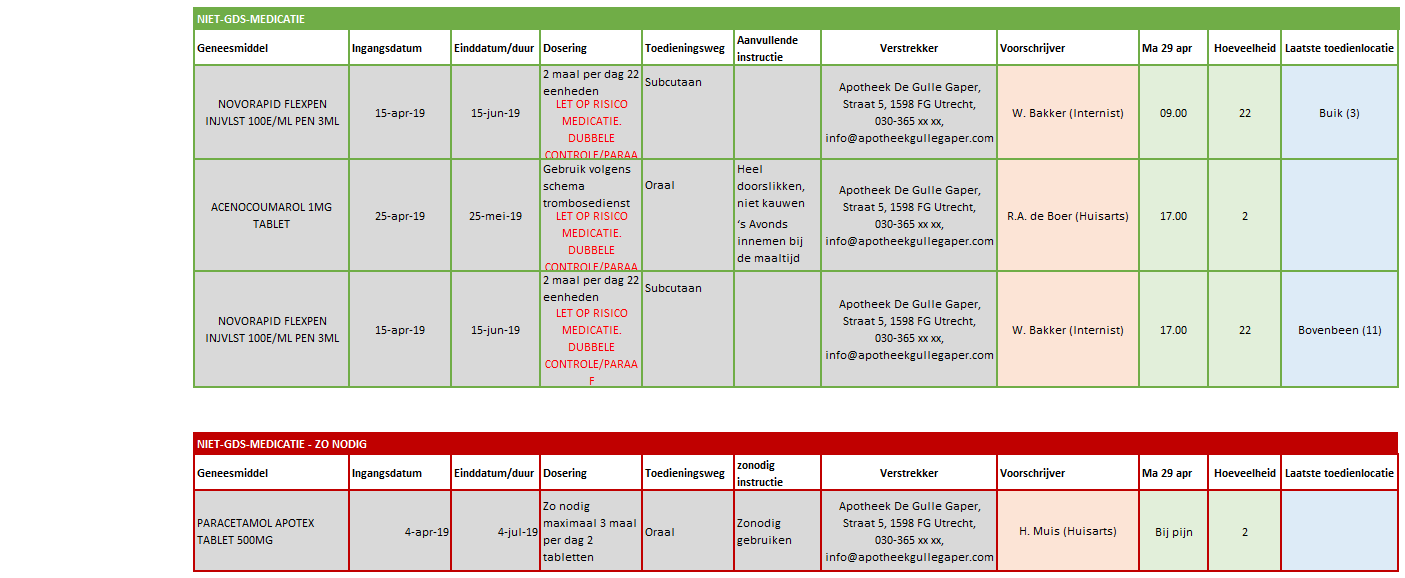
Figure 1: Example administration list.
| Nr | Heading | Dataset | ||
|---|---|---|---|---|
| 1 | Type | Medication Agreement | Administration Agreement | Variable-Dosing Regimen |
| 2 | Medicine | Agreed Medicine | Medicine To Be Dispensed | |
| 3 | Prescriber | Medication Agreement – Prescriber – Health Professional | ||
| 4 | Supplier | Administration Agreement – Supplier – Healthcare Provider | ||
| Author | Variable-Dosing Regimen – Author – Health Professional (conditional) | |||
| 5 | Start Date | Medication Agreement – Period Of Use | Administration Agreement – Period Of Use | Variable-Dosing Regimen – Period Of Use |
| 6 | End Date/Duration | Medication Agreement – Period Of Use | Administration Agreement – Period Of Use | Variable-Dosing Regimen – Period Of Use |
| 7 | Description | Instructions For Use – Description | ||
| Route Of Administration | Instructions For Use – Route Of Administration | |||
| Additional Instructions | Instructions For Use – Additional Instructions | |||
| Dose | Instructions For Use – Dosing Instructions – Dosage – Dose | |||
| Administration Time | Period Of Use – Dosing Instructions – Dosage – Administering Schedule – Administration Time | |||
| Administration Speed | Period Of Use – Dosing Instructions – Dosage – Administration Speed | |||
| Duration Of Administration | Period Of Use – Dosing Instructions – Dosage – Duration Of Administration | |||
| 8 | Prick-Patch Location | Medication Administration – Prick-Patch Location | ||
| 9 | Comment | Comment & Additional Information | ||
Table 1: Normative medication data in the administration list.
Functional specification
In the figures below the elements with normative data are presented. The other elements are not normative (not yet). The numbered elements in the table are normative; the other elements are optional (until further notice).
Heading and general
Remarks
In the comment field, for example, (limiting) health literacy competences (competences: literacy, calculation and digital skills) can be recorded, which may affect the medication administration. This part is for internal use and is not exchanged (not yet).
NB. This part is not normative yet. This data is derived from the internal information system of the organization.
GDS medication
GDS (medication distribution system, in Dutch: 'Geneesmiddel Distributie Systeem') is defined as a package in which medicaments are distributed in units per administration moment, labelled with the name of an individual patient/client (KNMP, 2011). If medication is discontinued or changed, it is desired that a warning signal is shown, with the comment that, possibly, the medication is in the GDS package, and an additional act may be required. Further elaboration is needed.
Non-GDS medication
Non-GDS medication is medication which, for various reasons, cannot be included in an automated medication distribution system. The distribution type 'non-GDS' is specified in the data element DistributionForm in the corresponding MVE. Furthermore, for non-GDS medication, the condition AsNeeded remains empty.
Non-GDS medication – as needed
Non-GDS medication as needed comprises separate medication which should be administered only in specific circumstances. The condition for administration of such medicament can be:
- a measured physiological value (plasma glucose level, body temperature, blood pressure);
- a symptom or other condition (headache, itching).
The distribution type 'non-GDS' is specified in the data element DistributionForm in the corresponding MVE. Furthermore, the condition AsNeeded is filled.
Building block instantiations
This paragraph describes which building block instantiations are related to the administration list. This includes only the ‘own’ building blocks (see 5.3.1) and only the latest, relevant instantiations (see 6.3.2).
Therapeutic and logistical building blocks
Compiling an administration list involves both therapeutic and logistical medication building blocks. Building blocks can be ‘overruled’ based on inference rules. All inference rules as described in Chapter 5 are applicable. In addition, in this paragraph, the inference rules for WDS are described. The inference rules do not apply to MTD, as medication administration is a procedure at a certain time point; in contrast, the MA, TA and MBG comprise agreements valid for a certain period of time. The logistical medication building block MVE is part of compiling the administration list, as this building block is needed to derive the type of distribution of the medication.
Administration list and inference rules
An administration list can be compiled, for example in the administrator’s information system, based on separate medication building blocks. The medication building blocks may be retrieved from the own information system or from other sources. The transaction Medication data (consulting/making available) is used for consulting and making available individual medication building blocks. In the transaction group Medication data (sending/receiving), separate medication building blocks can be send to another health professional or patient directly (without prior consultation); for example, in the case of discharge or on request of a fellow health professional (for example, a patient sees a GP or pharmacist outside the region; the GP or pharmacist requests the data by telephone and receives the data digitally). Both for Medication data sending/receiving and Medication data consulting/making available, data obtained from other sources is not allowed to be presented in the administration list.
Last relevant
Only the latest relevant medication building blocks (per pharmaceutical treatment) are part of the administration list. This paragraph provides a description of the latest relevant building blocks for an administration list comprising medication on prescription. Paragraph 6.3.2.1 describes the situation in which a variable-dosing regimen (WDS) is applicable. For WDS, the same inference rules apply as for MA. For compiling an administration list, the presence of a WDS is decisive for the instructions for use. All other situations in which WDS is not applicable, as described in paragraph 5.3.2, are applicable as well.
Query to identify the correct, relevant and future medication building blocks:
| Nr | Medication building block | Query Parameter | What should be filled in specifically? |
|---|---|---|---|
| 1 | MA, TA, WDS | Period Of Use | All medication that should be administered today and, therefore, is applicable: Day = T, startDate = T: 00:00:00 h, endDate = T: 23:59:59 h |
| 2 | MTD | Administration Period | Depending on medical relevance (for example, historical information on prick and patch locations may be relevant back to one week ago). For example, is one week is sufficient (another period may be chosen): Day = T, startDate = T-7 day |
| 3 | MVE | Dispense Period | Broad request (+/- one month) |
If all medication building blocks are collected, the following medication data, depending on the situation, can be used:
| Medication building block | Situation: only MA* | Situation: MA + TA | Situation: MA + TA + WDS |
|---|---|---|---|
| MA | Decisive** for compiling the administration list (number: 2, 5, 6, 7, 9, 10) | Data from the prescriber | Data from the prescriber |
| TA | - | Decisive** for compiling the administration list (number 2, 5, 6, 7, 9, 10) | Data from the supplier and medicine |
| WDS | - | - | Decisive** for compiling the administration list (number 5, 6, 7, 10) |
* Situation in which the pharmacist has not processed the MA yet. ** ‘Decisive’ indicates the data elements which are included in the functional requirements (numbers of the elements are provided between brackets).
Medication with prescription with a variable-dosing regimen
Most simple ‘happy flow’
The most simple ‘happy flow’ in a pharmaceutical treatment is stated below.
- MA – WDS – TA
In general, a pharmaceutical treatment starts with an MA. A TA specifies the MA. In the WDS, it is defined whether a variable-dosing regimen applies. In both the MA and the TA, the instruction ‘according to schedule thrombosis service’ is recorded. Then, in the WDS, the dosing schedule is specified. The three medication building blocks MA, TA and WDS are relevant and are part of compiling an administration list, as indicated in green below.
- MA – WDS– TA
A TA refers to the MA, which is specified by the TA. Also, a WDS refers to the MA, which is specified by the WDS. A WDS is always related to an MA.
New WSD
- MA – WDS – TA – WDS
The period of use of the WDS has ended and a new dosing schedule is created.
Change of WDS
- MA – WDS– TA – stop-WDS – WDS
The variable-dosing regimen (WDS) is changed.
Discontinuation of medication
- MA – WDS – TA – stop-MA
Medication with a WDS is discontinued by a stop of the MA.
Change of trade product
- MA – WDS – TA – stop-TA – TA
The trade product is changed; this affects the WDS.
Rules of overruling listed
Based on the descriptions/examples above, the following general rules for overruling apply. Those rules indicate which medication building block is relevant to incorporate and present in the administration list. Within a pharmaceutical treatment:
- A new MA overrules all previous, older therapeutical medication building blocks (MA, TA, WDS) related to the MA(s) which is/are stopped by the new MA.
- A new TA overrules the previous, older TA related to the same MA as the new TA.
- A new WDS overrules all previous, older WDSs related to the same MA as the new WDS.
Information systems and transactions
This chapter contains a list of all system roles and transactions that are referred to in the various process chapters.
The figure below (Figure 10) contains an overview of all information systems with their corresponding system roles. The system roles have been described in Table 3. The system roles associated with consulting/making available medication data and the medication overview are important for all information systems depending on the process in which they are used. The electronic prescribing system (EPS) also includes the system roles for receiving a voorstel verstrekkingsverzoek, sending a reply voorstel verstrekkingsverzoek and receiving a voorstel medicatieafspraak and, in an outpatient situation, for sending a prescription and receiving data on the processing of a prescription. In addition to the basic system roles, a XIS and PGD also include the system roles for sending a voorstel medicatieafspraak and a voorstel verstrekkingsverzoek, for sending data on gebruik and for receiving a reply voorstel verstrekkingsverzoek. The pharmacist information system (PIS) also includes, in addition to the basic system roles, the roles for sending a voorstel medicatieafspraak, sending a voorstel verstrekkingsverzoek and data on processing of the prescription, and receiving a prescription and reply voorstel verstrekkingsverzoek. Chapter 2 lists the main system roles per process.
| System role name | Abbr. | Description |
|---|---|---|
| MedicatieGegevensBeschikbaarstellend | MP-MGB | Making medication data available to fellow health professionals/patient |
| MedicatieGegevensRaadplegend | MP-MGR | Consulting medication data at fellow health professionals/patient |
| MedicatieGegevensSturend | MP-MGS | Sending data to fellow health professional/patient |
| MedicatieGegevensOntvangend | MP-MGO | Receiving medication data from fellow health professional/patient |
| MedicatieOverzichtBeschikbaarstellend | MP-MOB | Making medication overview available to fellow health professionals/patient |
| MedicatieOverzichtRaadplegend | MP-MOR | Consulting medication overview at fellow health professionals/patient |
| MedicatieOverzichtSturend | MP-MOS | Sturen medicatieoverzicht aan medebehandelaar/patiënt |
| MedicatieOverzichtOntvangend | MP-MOO | Receiving medication overview from fellow health professional/patient |
| VoorschriftSturend | MP-VOS | Sending a request to dispense medication (verstrekkingsverzoek) or process modifications to medicatieafspraak. |
| VoorschriftOntvangend | MP-VOO | Receiving verstrekkingsverzoek or request for processing of modifications to medicatieafspraak. |
| VoorschriftAfhandelingSturend | MP-VAS | Sending data on processing of medicatieafspraak and possibly verstrekkingsverzoek in toedieningsafspraak and/or medicatieverstrekking |
| VoorschriftAfhandelingOntvangend | MP-VAO | Receiving data on processing of medicatieafspraak and possibly verstrekkingsverzoek in toedieningsafspraak and/or medicatieverstrekking |
| VoorstelVerstrekkingsverzoekSturend | MP-VVS | Informing prescriber about voorstel new verstrekkingsverzoek, receiving reply to proposed verstrekkingsverzoek |
| VoorstelVerstrekkingsverzoekOntvangend | MP-VVO | Receiving new voorstel verstrekkingsverzoek, sending reply to voorstel verstrekkingsverzoek |
| VoorstelMedicatieafspraakSturend | MP-VMS | Informing prescriber about new voorstel or modified medicatieafspraak, receiving reply to proposed medicatieafspraak |
| VoorstelMedicatieafspraakOntvangend | MP-VMO | Receiving voorstel for new or modified medicatieafspraak, sending reply to voorstel medicatieafspraak |
Table 3 Overview system roles
Table 4 gives an overview of all transaction groups, transactions, corresponding system roles and building blocks exchanged with this transaction group. The names of the transaction groups and transactions link to the ART-DECOR publication which details per scenario which data elements are used.
| Transaction group | Transaction | System role | Building blocks |
|---|---|---|---|
| Medication data (PULL) | Making medication data available | MP-MGB | One or more: MA, VV, TA, MVE, MTD, MGB, WDS |
| Consulting medication data | MP-MGR | ||
| Medication data (PUSH) | Sending medication data | MP-MGS | One or more: MA, VV, TA, MVE, MTD, MGB, WDS |
| Receiving medication data | MP-MGO | ||
| Medication overview (PULL) | Making medication overview available | MP-MOB | MA, TA, MGB |
| Consulting medication overview | MP-MOR | ||
| Medication overview (PUSH) | Sending medication overview | MP-MOS | |
| Receiving medication overview | MP-MOO | ||
| Medication prescription (PUSH) | Sending medication prescription | MP-VOS | MA with or without VV, height, weight, renal function value |
| Receiving medication prescription | MP-VOO | ||
| Medication prescription processing (PUSH) | Sending data on processing of medication prescription | MP-VAS | TA with or without MVE |
| Receiving data on processing of medication prescription | MP-VAO | ||
| Proposal dispense request (PUSH) | Sending proposal dispense request | MP-VVS | VVV |
| Receiving proposal dispense request | MP-VVO | ||
| Reply proposal dispense request (PUSH) | Sending reply proposal dispense request | MP-VVO | AVVV |
| Receiving reply proposal dispense request | MP-VVS | ||
| Proposal medication agreement (PUSH) | Sending proposal medication agreement | MP-VMS | VMA with or without height, weight |
| Receiving proposal medication agreement | MP-VMO | ||
| Reply proposal medicationagreement (PUSH) | Sending reply proposal medicationagreement | MP-VMO | AVMA |
| Receiving reply proposal medicationagreement | MP-VMS |
Table 4 Overview transaction groups
Chapter 2 lists for each process only the relevant transactions per process step. The transaction groups are not included in this. Table 4 includes all transaction groups and transactions.
Functionality
This chapter describes indications for the functionality of an information system.
Filtering medication from 2nd/3rd line (all information systems)
An institution makes all its medication data (all building blocks) available. Not all data may be relevant for the receiving health professionals. Receiving information systems may therefore include a filter depending on the requirements of the specific health professional/patient. The list below (Table 5) includes medication types of which it has been determined that medicatietoediening during admission is relevant for health professionals outside the institution.
| ATC-code | Name |
|---|---|
| L01 | Cytostatics |
| L03AX03 | BCG vaccine, urology |
| L04AA23; L04AA25; L04AA26; L04AA33; L04AA34; L04AB; L04AC | Immunosuppressive agents |
| B03AC; B03XA; B06AC | Iron, erythropoietin, drugs used in angioedema |
| G03GA; G03GB02 | Gonadotropins (HCG, etc.), clomiphene |
| H01CA; H01CC; L02AE04 | Gonadorelin, hypothalamic hormones, triptorelin |
| J06; J07 | Immunoglobulins, vaccines |
| M03AX01 | Botulinum toxin |
| R03DX05 | Omalizumab |
| S01LA04; S01LA05; S01LA | OOphthalmological antineovascularisation agents |
Table 5 Types of clinical medication relevant for external health professionals
Making medication data available (all information systems)
All proprietary medication data may be made available when the patient has given his consent (in accordance with applicable laws and regulations). Data received from third parties copied (health care provider/patient) in one’s own information system and marked as ‘copy’, are not made available. The only exception is the transaction Medicatiegegevens (Medication data) PUSH and PULL and Medication overview PUSH and PULL, in which case third parties’ building blocks are made available on the condition that they include the original identification label (see also paragraph 5.1).
Notification date or dispense date (pharmacist information system)
The notification date and the actual dispense date may be recorded in the medicatieverstrekking. When a verstrekkingsverzoek is processed immediately and the patient picks up the medication that same day, both dates will be the same. When the patient picks up the medication one or several days later, different dates must be entered. The dispense date is the date on which the medicatieverstrekking has actually taken place. The request date is the time at which a pharmacy records an intended medicatieverstrekking. When medication is dispensed on several occasions as part of the same verstrekkingsverzoek (for example, when prescriptions are split), each medicatieverstrekking has its own request date. When an automated dispense system is being used, the dispense date is the time at which the patient picks up the medication from the dispense system. Until this time is available in the PIS, the time at which the medication is deposited in the automated dispense system may be used. See also paragraph 4.2.5.
Updating after system malfunction
After a system malfunction, the prescriber’s information system needs to determine whether changes have been made to medicatieafspraken. For use cases and rationale, see Discontinuation of medication by third parties and Two PRKs in a single medicamenteuze behandeling.
Construction for ‘once every 36 hours’ interval
When the dosing instruction reads ‘1 tablet every 36 hours’, this may normally be recorded in one medicatieafspraak (dose: 1 tablet, interval: 36 hours). However, when a information system does not go beyond an interval of, for example, 24 hours, another solution needs to be found.
Variation 1: Making use of an ‘as needed’ instruction with the criterion: ‘36 hours have passed since the last medicatietoediening’. This does however come with a certain amount of freedom that may not be desirable.
Variation 2: A repeating dosing schedule can be created, with alternation between administration in the morning, administration in the evening and a day without medicatietoediening:
Repeat period: 3 days Dosage instruction Sequence number: 1 Dosage duration: 1 day Dosage Dose per administration: 1 tablet Administration time: 9 am (per example, this could also be a part of the day) Sequence number: 2 Dosage duration: 1 day Dosage Dose per administration: 1 tablet Administration time: 9 pm (per example, this could also be a part of the day)
If it is not technically possible to choose a 36 hours interval, variation 2 applies.
EVS / HIS processing Regulation processing
The prescribing information system is informed by the pharmacist of all dispensings and changes in toedieningsafspraken through the transaction Afhandeling voorschrift (settlement prescription). This handling must be automatically processed in the prescribing information system. The prescriber only assesses the toedieningsafspraken and does not have to assess all the dispensings (verstrekkingen).
Examples dosages
See examples dosages 9 2.0.0 (both functional and technical effect in HL7v3 CDA and in FHIR)
Medicatiegebruik: use indicator, according to agreement indicator, stop type, period of use and dosing instructions
| Situation | 1. Medication is/will be used during period of use according to agreement | 2. Medication is/will be used during period of use, but not according to agreement | 3. Contrary to agreement, medication is/will not be used during period of use | 4. Medication is/will not be used during period of use, which is according to agreement | 5.Medication is used during period of use but it is unknown if this is according to agreement (selfmedication) |
| Use indicator (is this product being used) |
Yes | Yes | No | N/A (because this would lead to a double negative otherwise) | Yes |
|---|---|---|---|---|---|
| According to agreement indicator | No | No | No | Yes | - |
| Stoptype | N/A | N/A | Discontinuation or Stop | Definitve of Temporary | N/A |
| Stoptype depends if agreement is being stoped or dicontinued | Stoptype in acoordance with stop-agreement | ||||
| Period of use | In accordance with MA or TA | Max. 2 out of 3 | Max. 2 out of 3 | Max. 2 out of 3 | Max. 2 out of 3 |
| start date | The time at which the medicatiegebruik was started. | The time at which the deviating medicatiegebruik was/will be started. . | Contrary to agreement, medication is/will not be used during period of use | The time at which the medicatiegebruik was/will be discontinued. | The time at which the medicatiegebruik was started. |
| duration of medicatiegebruik | The intended duration of medicatiegebruik. | The intended duration of deviating medicatiegebruik. | The (intended) duration of non-use | The (intended) duration of non-use. | The intended duration of medicatiegebruik. |
| end date | The time at which the period of use ends (or has ended or will end). | The time at which the period of deviating use ends (or has ended or will end). | The time at which the use was/will be resumed (or is intended to resume). | The time at which the medicatiegebruik was/will be resumed (or is intended to resume). | The time at which the period of use ends (or has ended or will end). |
| Dosing instructions | In accordance with MA or TA | Required | N/A | N/A | Required |
| Medication was/will be used during the period of use according to the agreement, therefore dosage is known. | Medication was/will not be used according to the agreement, the actual dosage used must be communicated. | The medication was/will not be used, there is no dosage. | The medication was/will not be used, there is no dosage. | Dosage tha the patient has agreed upon himself |
Examples:
Situation 1a: Medication is / is used during the period of use according to agreement
Patient has been prescribed Ferrofumarate for anemia. Dosage is 1 tablet 3 times a day half a hour before eating, with a start date of June 4, 2018 and no end date.
On 10 June, the patient registers the medicatiegebruik for the past period (from 4 to 10 June).
| Situation 1a | |
|---|---|
| Medication | FERROFUMARATE 100MG TABLET |
| Use indicator | Yes |
| According to indicator agreement | Yes |
| Stoptype | Not applicable |
| Start date | 4 June 2018 |
| End date | 10 June 2018 |
| Dosing instruction | According agreement |
Situation 1b: Medication is / is used during the period of use according to agreement
Patient has been prescribed Ferrofumarate for anemia. Dosage is 1 tablet 3 times a day half an hour before eating, with a start date of June 4, 2018 and no end date.
On 10 June, the patient registers the medicatiegebruik for the past period (from 4 to 10 June).
| Situation 1b | |
|---|---|
| Medication | FERROFUMARATE 100MG TABLET |
| Use indicator | Yes |
| According to indicator agreement | Yes |
| Stoptype | Not applicable |
| Start date | 4 June 2018 |
| End date | - |
| Dosing instruction | According agreement |
Situation 2: Medication is / is used during the period of use, but not by agreement
Patient has been prescribed Ferrofumarate for anemia. Dosage is 1 tablet 3 times a day half an hour before eating, with a start date of June 4, 2018 and no end date.
The patient is on the road a lot during the day and forgets for a week to take the medicines for lunch. However, the tablets are taken in the morning and evening before eating.
| Situation 2 | |
|---|---|
| Medication | FERROFUMARATE 100MG TABLET |
| Use indicator | Yes |
| According to indicator agreement | No |
| Stoptype | Not applicable |
| Start date | 11 June |
| End date | 15 June |
| Dosing instruction | 1 tablet 2 times a day |
Situation 3: Contrary to the agreement, the medication is / is not used during the period of use
Patient has been prescribed Ferrofumarate for anemia. Dosage is 1 tablet 3 times a day half an hour before eating, with a start date of June 4, 2018 and no end date.
The patient goes on vacation for a long weekend and finds out too late that the medication is not packed. The patient will not take the tablets for the next few days and wants to record it.
| Situation 3 | |
|---|---|
| Medication | FERROFUMARATE 100MG TABLET |
| Use indicator | No |
| According to indicator agreement | No |
| Stoptype | Temporary |
| Start date | 20 July |
| End date | 23 July |
| Dosing instruction | Not applicable |
Situation 4: Medication is / is not used during the period of use and that is also by agreement
Patient has been prescribed Ferrofumarate for anemia. Dosage is 1 tablet 3 times a day half an hour before eating, with a start date of June 4, 2018.
On August 12, it turns out after checking that there is no longer anemia and the doctor stops the medication. The patient wants to register that she has stopped taking the medication and registers this herself on August 15.
| Situation 4 | |
|---|---|
| Medication | FERROFUMARATE 100MG TABLET |
| Use indicator | No |
| According to indicator agreement | Yes |
| Stoptype | Definite |
| Start date | 12 August 2018 |
| End date | - |
| Dosing instruction | Not applicable |
Situation 5: Medication is used during the period of use, but it is unknown whether this is by appointment or there is no appointment (self-care medication)
Patient has the flu and he takes paracetamol 500 mg from the local drug store for fever and pain. He has been taking 4 to 6 tablets a day for four days and expects to do this for another three days. He registers this on August 12 as medicatiegebruik.
| Situation 5 | |
|---|---|
| Medication | Paracetemol 500mg |
| Use indicator | Yes |
| According to indicator agreement | - |
| Stoptype | Not applicable |
| Start date | 9 August 2018 |
| End date | 7 days |
| Dosing instruction | 4 to 6 tablets a day |
Implementation of medication distribution system (GDS) fields
Background
More and more patients are receiving their medication through Individualized Distribution Forms (In dutch geindividualiseerde distributie vorm), with the pharmacist providing patients overview and organization of their drugs by preparing them in separate compartment units. This form of delivery took off when the Medicines Act in 2007 stipulated that in healthcare institutions without a pharmacy, the medication should be individually registered for the patients.
It is important for health professionals to know whether a patient is using GDS. In 2018, an analysis was conducted (under the direction of Z-Index, with health professionals) of the bottlenecks and wishes regarding the recording of a patient using GDS. Overall, it has emerged that it is desirable to be able to see whether a patient is using GDS at both the medication level and the patient level. For details see next paragraph.
The directions below provide guidance on how the Medication Process can support these needs.
Wishes of care providers
In 2018, an inventory was made with the user councils of Z-Index about the wishes regarding the recording and exchange of GDS. Below is an overview of these wishes.
Who
- All healthcare parties (from prescriber to operator)
What
- Determines which pharmacy delivers
- Pharmacy must know whether the change applies to the current roll or not
- Important for stopping previous medication at the start of GDS
- Evaluation of GDS patients for health insurer
- Other logistics processes towards home care / informal care
Why
- Determines which pharmacy delivers
- Pharmacy must know whether the change applies to the current roll or not
- Important for stopping previous medication at the start of GDS
- Evaluation of GDS patients for health insurer
- Other logistics processes towards home care / informal care
When
- When starting medication, in a pharmacy already before notification of prescription
- When transferring 1st / 2nd line
- When changing (incl. Stopping) medication
- When changing CiO data
Where
- When working in the patient's file, it should be clear that this characteristic applies to the patient concerned. This can be different for each type of care provider (patient file, medication file). The wish of public pharmacists is that they always have this characteristic available for a specific patient.
- On medication overview
Data elements Medication process
The building blocks for the Medication Process have the following fields that play a role in recording GDS.
| Buildingblock | Data-element nr | Description | Type of content |
|---|---|---|---|
| Medicatieafspraak | 23283 | Additional information | Additional information contains a list of details of the medicatieafsrpaak that are important for pharmacovigilance monitoring and completion by a pharmacist.
For example: "change in GDS immediately".. |
| Verstrekkingsverzoek | 22759 | Supplementary wishes | Logistical instructions important for the completion of the dispensing request by the pharmacist.
For example: "do not include in GDS" |
| Verstrekking | 20927 | Distribution form | Distribution form.
For example: GDS |
| 20924 | Consumption period | Stock for this duration | |
| 22500 | Registration date | The registration date is the time when a pharmacist records an intended dispensing | |
| 20272 | Date | The time of the dispensing. The date when the medicine is made available to the patient |
Application of data elements to support desired functionality for GDS patients
Recording that someone uses GDS
When working in the patient's file it is important to see that someone is using GDS. This applies to the pharmacy that supplies the GDS, as well as other care providers of the patient. It is therefore important that this information can be communicated with and shown to other health professionals.
As long as there is no patient characteristic with which GDS can be registered, it can be decided to derive this information from the GDS data that have been recorded with the medication. A possible handle for this is the fact that medication is included in the distribution module together with the Distribution form field in the verstrekking (dispensing).
- Step 1: if medication is included in the distribution module: fill in the Distribution form field in the verstrekking with 'GDS'.
- Step 2: Based on the Distribution form field, deduce that a patient is using GDS. The fact that a patient has a dispensing (vertrekking) where the Distribution form field is filled with "GDS" indicates that the patient is using GDS. This dispensing can be a private dipsenisn, or it can be dispensed from another pharmacy (insofar as this data is collected and recorded in such a way that the contents of the Distribution form field are reusable). The fact that a patient uses GDS can then be shown when working in the medication file or the patient file. In consultation with end users, it should be further determined where and how this is shown.
Recording why someone uses GDS
Users have indicated that it is desirable to be able to record why someone uses GDS. There are currently no structural fields available for this.
Record for medication that this must be in the GDS
Prescribers want to be able to indicate whether or not a medicine should be provided via GDS. This can be done as follows:
- Verstrekkingsverzoek, Additional wishes (aanvullende wensen) field. The code list for this field contains the item "not in GDS". This code list is thesaurus 2051 from file 902 in the G-Standard.
Establishing / exchanging the duration of a medication roll
The duration of the medication roll can be determined on the basis of the "duration of use" of the provision: the "duration of use" indicates the duration of a medication roll.
Establishing / exchanging whether a change in the medication should be effective immediately or not
It is desirable that a prescriber can indicate whether changes in the medication should be implemented immediately or that they can wait until the next roll change. The handles for this are as follows:
- Medicatieafspraak, Additional information (aanvullende informatie) field. The code list for this field contains two items that relate to changes in the GDS, namely "Change in GDS immediately" and "Change in GDS per roll change". This code list is thesaurus 2050 from file 902 in the G-Standard.
- It is important that the prescriber has insight into the duration of the roll and how long it will take before the current medication roll is used up. This can be deduced from:
- The verstrekking, period of use (verbruiksduur), see above. For this, the prescribing system must have access to the verstrekking data of previous verstrekkingen (dispensings).
- Verstrekking (dispensing), Registration Date (aanschrijfdatum) or Date (datum) field (or if this field is not filled but the Registration date field is, then the registration date.; if both are filled, Date is preferred). Based on this date and the consumption period, it is possible to calculate the date on which the current medication roll is used.
Reflections
Prescription without specified recipient (ambulatory)
In this paragraph, the term ‘prescription’ is used to indicate the message through which a patient may pick up a medicinal product from a supplier. A prescription contains a medicatieafspraak and a verstrekkingsverzoek.
In the Netherlands it is customary to digitally send prescriptions to a receiving pharmacy instead of having to collect prescriptions. This requires that the pharmacy is already known.
This paragraph considers whether more flexibility can be achieved in the logistical processing of prescriptions, without canceling out current advantages. This paragraph may be seen as a long-term vision that we aim towards.
Basic principles
When considering how to achieve flexibility in the processing of prescriptions, these basic principles have been formulated:
- The patient is free to choose where prescriptions are handled
- The current option for sending prescriptions remains possible.
- The information system must reuse the same functionality as much as possible.
- A prescription may only be dispensed once.
Since the methodology described below uses the existing method of sending prescriptions, the same legal rules and regulations apply to prescription validity. All discussions about electronic signatures are equally applicable to the current method of communicating prescriptions.
Details
A patient may choose from 3 options:
- A prescription is always sent to the same regular pharmacist.
- The patient indicates each time where the prescription has to be sent.
- The patient does not give any indication, and goes to a pharmacy which subsequently requests and processes the prescription.
Options 1 and 2 require a patient portal, in which the patient indicates his preference. For option 2, a patient may define a preferred pharmacy which would be at the top of the selection screen when the patient is asked where the prescription must be sent. If the patient does not give any indication, option 3 can be the only procedure.
The method partly uses a concept called “publish and subscribe”.
It is important that the pharmacist explains this principle to his customers.
Work process
A physician decides to create a prescription for a patient. This may be during a consultation or even when repeat prescriptions are requested. The physician registers the prescription in his prescribing system (EPS). The physician does not need to consider the pharmacy as the patient handles this through the portal.
After approval of the prescription, a central prescription index is updated. The EVS automatically sends a notification to this prescription registry to facilitate this. Here, the prescription index is seen as a separate registry with the data type “prescriptions”. It is always possible to consider whether or not this can be merged at a later time. Initially, atomic registration in the registry is being considered. However, at a later date, consideration could be given as to whether categorical registration would be sufficient.
It is important to realise that currently only a notification is given when a prescription is ready. After all, the prescription is still in the EVS database with the status “is available”. There is no prescription message yet.
The prescription registry knows from the patient’s preference settings what needs to be done with the notification:
- With the option ‘send to regular pharmacy’, a notification is sent to the subscription holder of the patient with the message that a prescription can be retrieved.
- With the option ‘patient indicates pharmacy’, the prescription registry sends a notification (e.g., SMS) to the patient. The prescription registry then waits until the patient uses a smartphone app or the patient portal to indicate to which pharmacy the notification should be sent.
- With the option ‘no indication’, the prescription registry does nothing. The patient does not have a regular subscription holder.
Processing by pharmacy
A receiving pharmacy can deduce from the transmitted notification signal (data type) that it refers to an open prescription. The signal also identifies who the patient is as well as the source of the prescription.
For patients who selected option 3 (no indication), the process starts now. The patient identifies himself and notifies the pharmacy that a prescription is ready at a certain institution. The pharmacist may look in the prescription registry to find out which EVS is involved.
The pharmacy information system asks the EVS source system to send the prescription of the respective patient. Since this is a request without medical content, it may even be submitted at lower trust levels.
Sending via EVS
If an EVS receives a request for “sending outstanding prescriptions”, the EVS will check whether prescriptions are indeed outstanding. This prevents a prescription from being retrieved several times. The address of the pharmacy is entered and the prepared prescription message is finally created. The prescription message is sent to the respective pharmacy.
If the prescription has been retrieved before, an error message is sent to the retrieving pharmacy announcing that the prescription is no longer available.
Sending prescriptions is the standard process and already customary for pushing prescriptions. The advantage of this is that the receiving pharmacy only needs to maintain one method for processing received prescriptions. It is noted again that the same legal rules and regulations continue to apply.
After sending a prescription, the EVS also sends a notification to the prescription registry to remove the entry of the outstanding prescription. This indicates that the respective prescription can no longer be retrieved.
Appendix References
General references
| Author(s) | Title | Version | Date (consultation) | Source | Organisation |
|---|---|---|---|---|---|
| NHG, KNMP, Z-index | Building blocks of the medication process | 2014 | Augustus 2015 | https://www.nhg.org/bouwstenen | NHG, KNMP, Z-index |
| ActiZ, KNMP, NVZA a.o | Safe principles in the medication chain | 2014 | August 2015 | http://www.knmp.nl/patiëntenzorg/samenwerking/brochure-veilige-principes-in-de-medicatieketen | ActiZ, KNMP, NVZA a.o. |
| Miscellaneous | Guideline on exchange of medication data in the medication chain | 25 April 2008 | August 2015 | Website KNMP | Miscellaneous |
| Paul Geels | Assessment of medication self-management (BEM) in care homes, Institute for Responsible Medication Use | February 2009 | http://www.instellingsapotheek.nl/downloads/rapporten/beoordeling_eigen_beheer_van_medicatie_in_verzorgingshuizen.pdf | Institute for responsible medicatiegebruik | |
| ActiZ | Safety in the medication chain | March 2012 | https://www.knmp.nl/downloads/brochure-veiligheid-in-de-medicatieketen.pdf | ActiZ, Miscellaneous | |
| KNMG | Guideline on electronic prescription | September 2013 | Website KNMG | KNMG |
Qualification scripts
Attachment: Document history
| Version | Date | Description |
|---|---|---|
| 0.95 | 15 July 2016 | Pilot version |
| 0.96 | 1 December 2016 | Paragraph 2.4 Process: Administer was adapted and C6: Sending/receiving administration data was added as a result. |
| 0.97 | 22 December 2016 | Paragraph 2.4, 4.3.1 review remarks incorporated. Paragraph 4.2.11 text made more precise. |
| 9.0.2 | 18 June 2017 | Conversion of the document to wiki. |
| 9.0.4 | September 2017 |
|
| 9.0.5 | January 2018 |
|
| 9.0.6 | May 2018 |
|
| 9.0.7 | December 2018 |
|
| 9.0.7 | July 2019 |
|
| 9.1.0 | 29 January 2020 |
|
| 9.1.0 | September 2020 |
|
| 9 2.0.0 bèta | 01 October 2021 | all changes see: *Releasenotes |
| 9 2.0.0 | 05 April 2022 | all changes see: *Releasenotes |
| 9 2.0.0 | 08 April 2022 | broken internal links FO corrected BITS MP-607 |
| 9 2.0.0 | 14 April 2022 | Changed images of uce case 4.1.38, 4.1.39 and 4.1.40, they showed GDS instead of WDS BITS MP-534 |
Appendix: Figures and tables
Figure 1 Building blocks - overview
Figure 2 Building blocks - coherence
Figure 3 Activity diagram - Medication process in general
Figure 4 Process steps and transactions - medication verification
Figure 5 Process steps and transactions - prescribing
Figure 6 Process steps and transactions - make available
Figure 7 Process steps and transactions - administer
Figure 8 Process steps and transactions - use
Figure 9 Example effective period
Figure 10 Overview of systems and system roles
Figure 11 Interaction diagram Prescribing without address
Table 1 Building blocks - description
Table 2 Informing versus (Active) making available
Table 3 Overview of system roles
Table 4 Overview of transaction groups
Table 5 Types of clinical medication relevant for outpatient care providers
- ↑ This document only uses the term medicatieafspraak, which therefore also indicates the clinical equivalent provisional medication order
- ↑ The verstrekkingsverzoek building block is not applicable in the clinical setting. Dispensing medication is handled in different ways in the clinical setting. Replenishment of, for example, a department’s supply is not considered a medicatieverstrekking, but rather an extension of the pharmacist’s stock. Medicatieverstrekking only takes place when the link between the medication and patient has been made. In clinical situations, administration often takes place immediately afterwards.
- ↑ 3,0 3,1 A provisional medication order, as it is used in hospitals, is both the request from the physician to the administrator to administer medication to the patient as a verstrekkingsverzoek to the pharmacist to ensure that the medication is available for the administrator. This last part corresponds to the medicatieafspraak and the verstrekkingsverzoek from the first line. In addition, the hospital pharmacist usually carries out a validation of the administration request (this creates the final medication order which is called a toedieningsafspraak here). The provisional medication order is therefore not the same as a proposal from, for example, from a nurse on the basis of a protocol that has not yet been approved by a physician.
- ↑ Use may have been preceded by medicatietoediening. Registration of medicatiegebruik, for example after medicatietoediening of rabies vaccinations or infusion is not obvious.
- ↑ From one-time to a certain period. The building block ‘Medicatieverbruik’ has not been detailed any further in this information standard.
- ↑ In practice several MAs (with different PRKs) are often combined into a single product instead of a single MA with underlying the composition.
- ↑ The patient may also verify his own medication. He then records medication use, see paragraph 2.5.4.1.
- ↑ This is about patients from a nursing home or a mental health institution who are going to the outpatient clinic of a hospital.
- ↑ This includes all health professionals who are authorised to prescribe: not only physicians, but also nurse practitioners and physician assistants, for example
- ↑ In the case of substitution, the existing MA is discontinued and a new MA is created under a new MBH.
- ↑ Information systems keep an audit trail. In case of a change, the existing MA is discontinued (new record) and a new MA with the change is then created (new record). By using the records of the audit trail, no major adaptations are needed for discontinuing a MA in most information systems.
- ↑ This does not mean that end users actually have to create two agreements. A user friendly presentation by the information system is desired.
- ↑ Correcting/canceling administration agreements happens in the same way. XIS is a generic term for a random (health care) information system. PHR=personal health records.
- ↑ XIS is a generic term for a random (health care) information system. PHR=personal health records.
- ↑ The patient may also request a repeat directly from the prescriber: see paragraph 2.5.6.
- ↑ In this document, the administration list means both the digital and the paper version, unless otherwise indicated. Sublist and checklist are synonyms.
- ↑ In a clinical situation, product may be taken from the department’s own supply, after which administration takes place immediately and the administration is recorded.
- ↑ XIS is a generic term for a random (health care) information system. PHR=personal health records.
- ↑ XIS is a generic term for a random (health care) information system. PHR=personal health records.
- ↑ See also paragraph 4.1.3.
- ↑ A similar situation occurs when the pharmacist receives a fax.