BgZ:V1.0 BgZ 2017 Technical IG: verschil tussen versies
Links aangepast naar 1.2.2. |
|||
| (11 tussenliggende versies door 2 gebruikers niet weergegeven) | |||
| Regel 1: | Regel 1: | ||
__NUMBEREDHEADINGS__ | __NUMBEREDHEADINGS__ | ||
__NOINDEX__ | __NOINDEX__ | ||
{{DISPLAYTITLE:BgZ Technical Implementation Guide}} | {{DISPLAYTITLE:BgZ Technical Implementation Guide}} | ||
__TOC__ | __TOC__ | ||
=Introduction= | =Introduction= | ||
See the [[BgZ:V1.0_BgZ_MSZ_Informatiestandaard|Functional specifcation]] for context. | |||
=Actors involved= | =Actors involved= | ||
| Regel 32: | Regel 29: | ||
System roles are further defined through the Healthcare Information Exchange model of "Registratie aan de Bron" ("Register at the source"). | System roles are further defined through the Healthcare Information Exchange model of "Registratie aan de Bron" ("Register at the source"). | ||
[[Bestand:Healthcare Information Exchange model v1.0.png|Healthcare Information Exchange model v1.0.png]] | [[Bestand:Healthcare Information Exchange model v1.0.png|Healthcare Information Exchange model v1.0.png]] | ||
BgZ-compliant systems should use | BgZ-compliant systems should use HCIMs with appropriate metadata, not only in exchange but also when recording and processing data. Since HCIMs do not have many mandatory items, a sparsely filled BgZ woudl easily be compliant for exchange. The need however is to be able to record, exchange and process the full HCIMs. Therefore, it is a requirement for a Storing System to register the full HCIMs, for an Exchanging System to provide the full HCIMs and for a Processing System to process the entire zib. | ||
Further requirements are specified in: | Further requirements are specified in: | ||
| Regel 42: | Regel 39: | ||
The [[BgZ:V1.0_BgZ_MSZ_Informatiestandaard#Metagegevens|functional description]] requires metadata for the BgZ. This chapter outlines the technical implementation of those metadata. | The [[BgZ:V1.0_BgZ_MSZ_Informatiestandaard#Metagegevens|functional description]] requires metadata for the BgZ. This chapter outlines the technical implementation of those metadata. | ||
==Document metadata== | ==Document metadata== | ||
For CDA Documents, the following metadata are mandatory (i.e. must always be present in the BgZ). | |||
* ClinicalDocument/id: the identifier of the entire BgZ document. | |||
* ClinicalDocument/effectiveTime: the date and time the BgZ was generated. | |||
* ClinicalDocument/recordTarget/patientRole/id: the id of the Patient who is subject of this BgZ. | |||
* ClinicalDocument/author/assignedAuthor/representedOrganization/id: the BgZ will rarely have a human as author. Nevertheless, author is required in CDA. Multiple authors are allowed. One should be the same as the custodian for the BgZ. | |||
* ClinicalDocument/custodian/assignedCustodian/representedCustodianOrganization/id: the organization who has generated this BgZ. | |||
These items loosely correspond with the BasicElements: | |||
* IdentificationNumber | |||
* DateTime | |||
* Subject | |||
* Author | |||
* Information Source | |||
They are not the same, since the BasicElements are about HCIMs and not documents. When HCIM do not carry sufficient metadata (see: [[#Historical and Absent Metadata|Historical and Absent Metadata]] below), the document metadata can provide the necessary context. | |||
==HCIM (zib) metadata== | ==HCIM (zib) metadata== | ||
===Basic Elements=== | ===Basic Elements=== | ||
| Regel 107: | Regel 119: | ||
====Historical and Absent Metadata==== | ====Historical and Absent Metadata==== | ||
For historical data, the appropriate metadata may simply no be available. In those cases those metadata items MUST NOT be exchanged, unless they are also persisted in the source system. In cases where | For historical data, the appropriate metadata may simply no be available. In those cases those metadata items MUST NOT be exchanged, unless they are also persisted in the source system. In cases where HCIMs are received from another organization, and metadata are absent or incomplete, the metadata SHOULD NOT be exchanged when sending to yet another organization (i.e. one should not invent identifiers when one is not the original source of the data). | ||
===Metadata table=== | ===Metadata table=== | ||
| Regel 392: | Regel 404: | ||
==Provenance== | ==Provenance== | ||
Every data item is a statement, an assertion of a fact, recorded by someone somewhere. This original source should be retained when integrating data from multiple sources. The source can be: | Every data item is a statement, an assertion of a fact, recorded by someone somewhere. This original source should be retained when integrating data from multiple sources. The source can be: | ||
# An individual as Information Source of the statement. Examples: | # An individual as Information Source of the statement. This is the InformationSource from the zib. In CDA of FHIR this is the corresponding element when a person is populating this element. Examples: | ||
#* a health professional makes a diagnosis | #* a health professional makes a diagnosis | ||
#* a patient reports alcohol and tobacco use | #* a patient reports alcohol and tobacco use | ||
# An organization as Information Source of the statement. Examples: | # An organization as Information Source of the statement. This is the InformationSource from the zib. In CDA of FHIR this is the corresponding element when an organization person is populating this element. Examples: | ||
#* a lab reports certain chemical or biological measurements on a specimen | #* a lab reports certain chemical or biological measurements on a specimen | ||
# A XIS as the information source. Examples: | # A XIS as the information source. This is the custodian a CDA document, and the XIS from which the HCIM was retrieved in FHIR (possibly other elements are suitable, as in XDS based exchanges or FHIR Documents). in Examples: | ||
#* a BgZ is received from a XIS, and the contact persons of the patient are listed without further author or source; | #* a BgZ is received from a XIS, and the contact persons of the patient are listed without further author or source; | ||
#* a record is retrieved from a XIS, and the historical problems in the problem list do not have further author or source; | #* a record is retrieved from a XIS, and the historical problems in the problem list do not have further author or source; | ||
| Regel 414: | Regel 426: | ||
* when a record is received from B and C, and A is listed as the information source in both, assume the most recently received record is most recent one. | * when a record is received from B and C, and A is listed as the information source in both, assume the most recently received record is most recent one. | ||
A Processing System can flag such conflicting records. When in doubt, a medical professional should decide, possibly after consulting colleagues. | A Processing System can flag such conflicting records. When in doubt, a medical professional should decide, possibly after consulting colleagues. | ||
=Implementation technologies= | |||
=FHIR profiles= | ==FHIR profiles== | ||
The [[MedMij:V2020.01/FHIR_BGZ_2017#List_of_profiles|FHIR profiles defined for MedMij]] are also used for the BgZ. | The [[MedMij:V2020.01/FHIR_BGZ_2017#List_of_profiles|FHIR profiles defined for MedMij]] are also used for the BgZ. | ||
=CDA templates= | ==CDA templates== | ||
The entire publication can be found at [https://decor.nictiz.nl/pub/bgz2017/bgz2017-html- | The entire publication can be found at [https://decor.nictiz.nl/pub/bgz2017/bgz2017-html-20220506T101050/ Basisgegevensset Zorg 2017 (BgZ)] | ||
The following CDA templates are used for the BgZ 2017. | The following CDA templates are used for the BgZ 2017. | ||
| Regel 430: | Regel 442: | ||
| 0 | | 0 | ||
| colspan="2" | BgZ | | colspan="2" | BgZ | ||
| [https://decor.nictiz.nl/pub/bgz2017/bgz2017-html- | | [https://decor.nictiz.nl/pub/bgz2017/bgz2017-html-20220506T101050/tmp-2.16.840.1.113883.2.4.3.11.60.42.10.1-2021-11-29T000000.html Template CDA Basisgegevensset Zorg 2017 (BgZ)] | ||
|- | |- | ||
| colspan="2" | 1. Demografie en identificatie | | colspan="2" | 1. Demografie en identificatie | ||
| Demographics and identification | | Demographics and identification | ||
| [https://decor.nictiz.nl/pub/bgz2017/bgz2017-html- | | [https://decor.nictiz.nl/pub/bgz2017/bgz2017-html-20220506T101050/tmp-2.16.840.1.113883.2.4.3.11.60.42.10.3-2021-11-29T000000.html CDArecordTargetSDTC-NL] | ||
|- | |- | ||
| | | | ||
| Regel 443: | Regel 455: | ||
| colspan="2" | 2. Financiële informatie | | colspan="2" | 2. Financiële informatie | ||
| Financial information | | Financial information | ||
| [http://decor.nictiz.nl/pub/bgz2017/bgz2017-html- | | [http://decor.nictiz.nl/pub/bgz2017/bgz2017-html-20220506T101050/tmp-2.16.840.1.113883.2.4.3.11.60.42.10.19-2017-02-05T000000.html BgZ2017Betaler] | ||
|- | |- | ||
| | | | ||
| Regel 452: | Regel 464: | ||
| colspan="2" | 3. Behandelrestricties | | colspan="2" | 3. Behandelrestricties | ||
| Treatment Directives | | Treatment Directives | ||
| [http://decor.nictiz.nl/pub/bgz2017/bgz2017-html- | | [http://decor.nictiz.nl/pub/bgz2017/bgz2017-html-20220506T101050/tmp-2.16.840.1.113883.2.4.3.11.60.42.10.4-2017-10-24T000000.html BgZ2017TreatmentDirectives] | ||
|- | |- | ||
| rowspan="2" | | | rowspan="2" | | ||
| Regel 465: | Regel 477: | ||
| colspan="2" | 4. Contactpersonen | | colspan="2" | 4. Contactpersonen | ||
| Contact persons | | Contact persons | ||
| [https://decor.nictiz.nl/pub/bgz2017/bgz2017-html- | | [https://decor.nictiz.nl/pub/bgz2017/bgz2017-html-20220506T101050/tmp-2.16.840.1.113883.2.4.3.11.60.42.10.3-2021-11-29T000000.html CDArecordTargetSDTC-NL] | ||
|- | |- | ||
| | | | ||
| Regel 474: | Regel 486: | ||
| colspan="2" | 5. Functionele status | | colspan="2" | 5. Functionele status | ||
| Functional Status | | Functional Status | ||
| [http://decor.nictiz.nl/pub/bgz2017/bgz2017-html- | | [http://decor.nictiz.nl/pub/bgz2017/bgz2017-html-20220506T101050/tmp-2.16.840.1.113883.2.4.3.11.60.42.10.5-2017-10-24T000000.html BgZ2017FunctionalStatus] | ||
|- | |- | ||
| | | | ||
| [https://zibs.nl/wiki/FunctioneleOfMentaleStatus-v3.1(2017NL) | | [https://zibs.nl/wiki/FunctioneleOfMentaleStatus-v3.1(2017NL) FunctioneleOfMentaleStatus] | ||
| [https://zibs.nl/wiki/FunctionalOrMentalStatus-v3.1(2017EN) FunctionalOrMentalStatus] | | [https://zibs.nl/wiki/FunctionalOrMentalStatus-v3.1(2017EN) FunctionalOrMentalStatus] | ||
| | | | ||
| Regel 483: | Regel 495: | ||
| colspan="2" | 6. Klachten en diagnoses | | colspan="2" | 6. Klachten en diagnoses | ||
| Complaints and diagnoses | | Complaints and diagnoses | ||
| [https://decor.nictiz.nl/pub/bgz2017/bgz2017-html- | | [https://decor.nictiz.nl/pub/bgz2017/bgz2017-html-20220506T101050/tmp-2.16.840.1.113883.2.4.3.11.60.42.10.18-2018-01-20T000000.html Voorgeschiedenis] | ||
[http://decor.nictiz.nl/pub/bgz2017/bgz2017-html- | [http://decor.nictiz.nl/pub/bgz2017/bgz2017-html-20220506T101050/tmp-2.16.840.1.113883.2.4.3.11.60.42.10.6-2017-10-24T000000.html BgZ2017ComplaintsAndDiagnoses] | ||
|- | |- | ||
| | | | ||
| Regel 493: | Regel 505: | ||
| colspan="2" | 7. Sociale anamnese | | colspan="2" | 7. Sociale anamnese | ||
| Social anamnesis | | Social anamnesis | ||
| [http://decor.nictiz.nl/pub/bgz2017/bgz2017-html- | | [http://decor.nictiz.nl/pub/bgz2017/bgz2017-html-20220506T101050/tmp-2.16.840.1.113883.2.4.3.11.60.42.10.7-2017-10-24T000000.html BgZ2017SocialAnamesis] | ||
|- | |- | ||
| rowspan="5" | | | rowspan="5" | | ||
| Regel 518: | Regel 530: | ||
| colspan="2" | 8. Waarschuwingen | | colspan="2" | 8. Waarschuwingen | ||
| Alerts | | Alerts | ||
| [http://decor.nictiz.nl/pub/bgz2017/bgz2017-html- | | [http://decor.nictiz.nl/pub/bgz2017/bgz2017-html-20220506T101050/tmp-2.16.840.1.113883.2.4.3.11.60.42.10.8-2017-10-24T000000.html BgZ2017Alerts] | ||
|- | |- | ||
| | | | ||
| Regel 527: | Regel 539: | ||
| colspan="2" | 9. Allergieën | | colspan="2" | 9. Allergieën | ||
| Allergies | | Allergies | ||
| [http://decor.nictiz.nl/pub/bgz2017/bgz2017-html- | | [http://decor.nictiz.nl/pub/bgz2017/bgz2017-html-20220506T101050/tmp-2.16.840.1.113883.2.4.3.11.60.42.10.9-2017-10-24T000000.html BgZ2017Allergies] | ||
|- | |- | ||
| | | | ||
| Regel 536: | Regel 548: | ||
| colspan="2" | 10. Medicatie | | colspan="2" | 10. Medicatie | ||
| Medication | | Medication | ||
| [http://decor.nictiz.nl/pub/bgz2017/bgz2017-html- | | [http://decor.nictiz.nl/pub/bgz2017/bgz2017-html-20220506T101050/tmp-2.16.840.1.113883.2.4.3.11.60.42.10.10-2017-10-24T000000.html BgZ2017Medication] | ||
|- | |- | ||
| rowspan="3" | | | rowspan="3" | | ||
| Regel 553: | Regel 565: | ||
| colspan="2" | 11. Medische hulpmiddelen | | colspan="2" | 11. Medische hulpmiddelen | ||
| Medical devices | | Medical devices | ||
| [http://decor.nictiz.nl/pub/bgz2017/bgz2017-html- | | [http://decor.nictiz.nl/pub/bgz2017/bgz2017-html-20220506T101050/tmp-2.16.840.1.113883.2.4.3.11.60.42.10.11-2017-10-24T000000.html BgZ2017MedicalDevices] | ||
|- | |- | ||
| | | | ||
| Regel 562: | Regel 574: | ||
| colspan="2" | 12. Vaccinaties | | colspan="2" | 12. Vaccinaties | ||
| Immunizations | | Immunizations | ||
| [http://decor.nictiz.nl/pub/bgz2017/bgz2017-html- | | [http://decor.nictiz.nl/pub/bgz2017/bgz2017-html-20220506T101050/tmp-2.16.840.1.113883.2.4.3.11.60.42.10.12-2021-10-28T000000.html BgZ2017Immunizations] | ||
|- | |- | ||
| | | | ||
| Regel 571: | Regel 583: | ||
| colspan="2" | 13. Vitale functies | | colspan="2" | 13. Vitale functies | ||
| Vital signs | | Vital signs | ||
| [http://decor.nictiz.nl/pub/bgz2017/bgz2017-html- | | [http://decor.nictiz.nl/pub/bgz2017/bgz2017-html-20220506T101050/tmp-2.16.840.1.113883.2.4.3.11.60.42.10.13-2017-10-24T000000.html BgZ2017VitalSigns] | ||
|- | |- | ||
| rowspan="3" | | | rowspan="3" | | ||
| Regel 588: | Regel 600: | ||
| colspan="2" | 14. Uitslagen | | colspan="2" | 14. Uitslagen | ||
| Results | | Results | ||
| [http://decor.nictiz.nl/pub/bgz2017/bgz2017-html- | | [http://decor.nictiz.nl/pub/bgz2017/bgz2017-html-20220506T101050/tmp-2.16.840.1.113883.2.4.3.11.60.25.10.52-2021-10-27T000000.html Laboratoryspecialtysection] | ||
|- | |- | ||
| | | | ||
| Regel 597: | Regel 609: | ||
| colspan="2" | 15. Verrichtingen | | colspan="2" | 15. Verrichtingen | ||
| Procedures | | Procedures | ||
| [http://decor.nictiz.nl/pub/bgz2017/bgz2017-html- | | [http://decor.nictiz.nl/pub/bgz2017/bgz2017-html-20220506T101050/tmp-2.16.840.1.113883.2.4.3.11.60.42.10.15-2017-10-24T000000.html BgZ2017Procedures] | ||
|- | |- | ||
| | | | ||
| Regel 606: | Regel 618: | ||
| colspan="2" | 16. Contacten | | colspan="2" | 16. Contacten | ||
| Encounters | | Encounters | ||
| [http://decor.nictiz.nl/pub/bgz2017/bgz2017-html- | | [http://decor.nictiz.nl/pub/bgz2017/bgz2017-html-20220506T101050/tmp-2.16.840.1.113883.2.4.3.11.60.42.10.16-2017-10-24T000000.html BgZ2017Encounters] | ||
|- | |- | ||
| | | | ||
| Regel 615: | Regel 627: | ||
| colspan="2" | 17. Zorgplan | | colspan="2" | 17. Zorgplan | ||
| Care plan | | Care plan | ||
| [http://decor.nictiz.nl/pub/bgz2017/bgz2017-html- | | [http://decor.nictiz.nl/pub/bgz2017/bgz2017-html-20220506T101050/tmp-2.16.840.1.113883.2.4.3.11.60.42.10.17-2021-07-09T172550.html BgZ2017CarePlan] | ||
|- | |- | ||
| | | | ||
| Regel 635: | Regel 647: | ||
|} | |} | ||
=Infrastructure= | |||
This standard does not mandate a particular infrastructure, and therefore does not specify details for such an infrastructure. | |||
Useful materials from other parties can be found on: | |||
* [https://taskforce-samen-vooruit.nl/downloads/ Taskforce Samen Vooruit] | |||
=Release notes= | =Release notes= | ||
Huidige versie van 3 okt 2022 om 10:06
__NUMBEREDHEADINGS__
Introduction
See the Functional specifcation for context.
Actors involved
| Persons | Systems | ||
|---|---|---|---|
| Name | Description | Name | Description |
| Referring medical specialist | Medical specialist who sends a BgZ along with a referral | EHR | Electronic health record |
| Receiving medical specialist | Medical specialist receives a BgZ along with a referral | EHR | Electronic health record |
Systems and system roles
System roles are further defined through the Healthcare Information Exchange model of "Registratie aan de Bron" ("Register at the source").
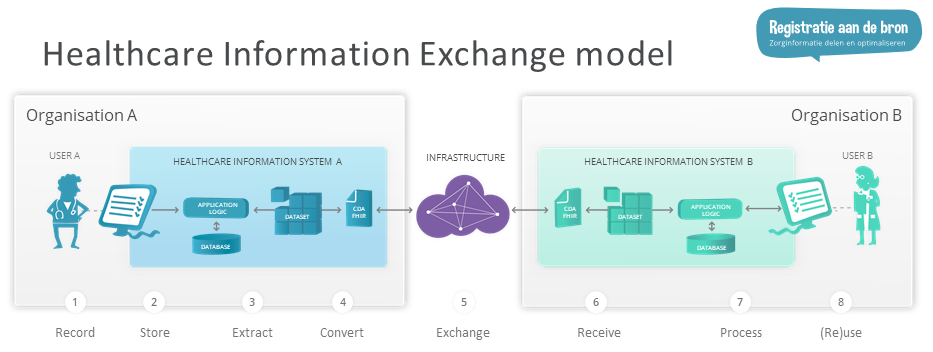 BgZ-compliant systems should use HCIMs with appropriate metadata, not only in exchange but also when recording and processing data. Since HCIMs do not have many mandatory items, a sparsely filled BgZ woudl easily be compliant for exchange. The need however is to be able to record, exchange and process the full HCIMs. Therefore, it is a requirement for a Storing System to register the full HCIMs, for an Exchanging System to provide the full HCIMs and for a Processing System to process the entire zib.
BgZ-compliant systems should use HCIMs with appropriate metadata, not only in exchange but also when recording and processing data. Since HCIMs do not have many mandatory items, a sparsely filled BgZ woudl easily be compliant for exchange. The need however is to be able to record, exchange and process the full HCIMs. Therefore, it is a requirement for a Storing System to register the full HCIMs, for an Exchanging System to provide the full HCIMs and for a Processing System to process the entire zib.
Further requirements are specified in:
- the HCIM definitions and technical artefacts
- the Qualification Scenario's
- in the Metadata section below.
Metadata
The functional description requires metadata for the BgZ. This chapter outlines the technical implementation of those metadata.
Document metadata
For CDA Documents, the following metadata are mandatory (i.e. must always be present in the BgZ).
- ClinicalDocument/id: the identifier of the entire BgZ document.
- ClinicalDocument/effectiveTime: the date and time the BgZ was generated.
- ClinicalDocument/recordTarget/patientRole/id: the id of the Patient who is subject of this BgZ.
- ClinicalDocument/author/assignedAuthor/representedOrganization/id: the BgZ will rarely have a human as author. Nevertheless, author is required in CDA. Multiple authors are allowed. One should be the same as the custodian for the BgZ.
- ClinicalDocument/custodian/assignedCustodian/representedCustodianOrganization/id: the organization who has generated this BgZ.
These items loosely correspond with the BasicElements:
- IdentificationNumber
- DateTime
- Subject
- Author
- Information Source
They are not the same, since the BasicElements are about HCIMs and not documents. When HCIM do not carry sufficient metadata (see: Historical and Absent Metadata below), the document metadata can provide the necessary context.
HCIM (zib) metadata
Basic Elements
The 2017 HCIM all have the following implicit Basic Elements which provide metadata:
IdentificationNumber
Identification is usually required for deduplication and unambiguous identification of data. Most HCIMs do not provide identification, so Problems, Procedures, Medication etc. need an IdentificationNumber. Some HCIM (such as Patient) already have an IdentificationNumber, and need no additional metadata. For some (Contact Persons) use of BSN is not permitted, and providing another non-unique id is not desirable (How could one keep that consistent over organizations?) Identification only makes sense when data has a single clear origin. In some other cases, identification is of little added value. I.e. for Body Weight, the datetime when it was recorded should suffice. To provide an additional "Body Weight id" to deduplicate Body Weight records is of little added value.
DateTime
Quite often HCIMs already have some DateTime. The relevant datetime usually is that of the event itself (say a surgery or medication administration), not of the recording of the event. For the BgZ, all relevant datetimes are already part of the HCIM. The others do not have to be provided (i.e., a Patients Contact Person carries no relevant datetime).
Subject
Subject is always implicit in the BgZ. It is always the Patient itself, except for Contact Persons and Health Professionals/Organizations, where the entire HCIM relates to the Patient and the data items therein of course pertain to the Contact Persons and Health Professionals/Organizations in question. The subject thus does not have to be separately stored, assuming the data will be stored in a Patient Record anyway, though exchange formats (certainly FHIR) may require the Patient resource to be part of exchanged data.
Author and Information Source
The basic elements state:
- Information Source: The person who provided the information and ensures the correctness of it... The source of information does not have to be the author of the information, who in that case is only instrumental in capturing the information.
- Author: The person who has recorded the information.
In the context of the BgZ, when the information source is an healthcare professional (or organization), the person who actually entered or recorded the information (data enterer, capturer) etc. is never relevant. A healthcare professional as information source is is responsible for the medical data, and the organization is responsible for the proper recording and exchange: this should never be an individuals responsibility. The only case where author can be relevant is when the patient is the source of information. In such cases, optionally, the "Patient as author" is provided, and responsible healthcare professional as Information Source.
In CDA, there are the following header participants in a CDA Document:
- Author: A party that originates the Act and therefore has responsibility for the information given in the Act.
- DataEnterer: A person entering the data into the originating system.
Therefore, HCIM:Author corresponds more with CDA:dataEnterer and HCIM:InformationSource corresponds more with Author. Be aware of this collision of terminology!
FHIR does not always clearly distinguish Author and Information Source. FHIR Observations, for instance, have a single 'performer' which can be a Patient or a Professional. It is often not be possible (nor needed) to exchange both Information Source and Author in FHIR. Usually the correct reading of the 'responsible person' present in FHIR is Information Source.
For almost all records either is relevant. Some listed exceptions are Patient, Contact Person, Payer. In other cases information which typically originates with the Patient, and which is recorded in the anamnesis, the preferred Basic Element to store the fact that the data is provided by the Patient is Information Source. When the information typically originates from a Healthcare Professional, such as a diagnosis, that professional is the Information Source. It is never required to provide both an Author and an Information Source when both are the same, i.e. it is not necessary to state that a diagnosis has the same doctor as Information Source AND Author, or that the Patient is Information Source AND Author of an Alcohol Use statement.
System Requirements for Metadata
| Required |
|
|---|---|
| Optional | This is for metadata items which are not crucial to interoperability. I.e. when a measurement already has an Author, the Information Source is not relevant. |
| Not relevant | For certain HCIMs the metadata items are not important. I.e. for the contact persons of a Patient, it is not relevant who or when recorded this. |
| Named data elements | Often the metadata items from the Basic Elements are already present in the HCIM itself. In this case these data items double as metadata, i.e. there is no need for a separate metadata item. All those metadata items are considered Required. |
| Other / textual | On other cases textual clarification is given in the table. |
|
Example Procedure For a Procedure which is recorded in a Storing System (i.e. which originates in the organization), the system:
|
|
Example LivingSituation For a LivingSituation which is recorded in a Storing System (i.e. which originates in the organization), the system must:
|
Historical and Absent Metadata
For historical data, the appropriate metadata may simply no be available. In those cases those metadata items MUST NOT be exchanged, unless they are also persisted in the source system. In cases where HCIMs are received from another organization, and metadata are absent or incomplete, the metadata SHOULD NOT be exchanged when sending to yet another organization (i.e. one should not invent identifiers when one is not the original source of the data).
Metadata table
Metadata are often already present in the HCIMs themselves: in that case there is no need to add them to the HCIM again. In other cases, the metadata is not relevant. The table below lists the various Basic Elements against the HCIMs.
| Id | Zib | HCIM | IdentificationNumber | DateTime | Author | Subject | InformationSource |
|---|---|---|---|---|---|---|---|
| NL-CM:0.1.1 | Patient | Patient | PatientIdentificationNumber | DateOfBirth | Not Relevant | Patient | Not Relevant |
| NL-CM:1.1.1 | Betaler | Payer | BankCode+AccountNumber OR IdentificationNumber+InsurantNumber | StartDateTime+EndDateTime | Not Relevant | Patient | Not Relevant |
| NL-CM:2.1.1 | BehandelAanwijzing | TreatmentDirective | Required | VerificationDate | Optional | Patient | Patient OR patient's authorized representative |
| NL-CM:7.15.1 | Wilsverklaring | AdvanceDirective | Required | LivingWillDate | Optional | Patient | Patient OR patient's authorized representative |
| NL-CM:3.1.1 | Contactpersoon | Contactperson | Not Relevant | Not Relevant | Not Relevant | Contactpersoon | Not Relevant |
| NL-CM:4.26.1 | FunctioneleOfMentaleStatus | FunctionalOrMentalStatus | Required | StatusDate | Optional | Patient | Required |
| NL-CM:5.1.1 | Probleem | Problem | Required | ProblemStartDate | Optional | Patient | Required |
| NL-CM:7.8.1 | Woonsituatie | LivingSituation | Optional | Not Relevant | Not Relevant | Patient | Optional |
| NL-CM:7.4.1 | DrugsGebruik | DrugUse | Required | StartDate | Optional | Patient | Required |
| NL-CM:7.3.1 | AlcoholGebruik | AlcoholUse | Required | StartDate | Optional | Patient | Required |
| NL-CM:7.2.1 | TabakGebruik | TobaccoUse | Required | StartDate | Optional | Patient | Required |
| NL-CM:7.11.1 | Voedingsadvies | NutritionAdvice | Required | Not Relevant | Optional | Patient | Required |
| NL-CM:8.3.1 | Alert | Alert | Required | StartDateTime | empty OR equal to Source | Patient | Required |
| NL-CM:8.2.1 | AllergieIntolerantie | AllergyIntolerance | Required | StartDateTime | empty OR equal to Source | Patient | Required |
| NL-CM:9.6.9580 | Medicatieafspraak | MedicationAgreement | Required | MedicationAgreementDateTime | empty OR equal to Source | Patient | Prescriber::HealthProfessional |
| NL-CM:9.8.20132 | Toedieningsafspraak | AdministrationAgreement | Required | AdministrationAgreementDateTime | empty OR equal to Source | Patient | Supplier::HealthProfessional |
| NL-CM:9.11.21338 | MedicatieGebruik | MedicationUse2 | Required | MedicationUseDateTime | empty OR equal to Source | Patient | Prescriber::HealthProfessional |
| NL-CM:10.1.1 | MedischHulpmiddel | MedicalDevice | ProductID | StartDate | empty OR equal to Source | Patient | HealthProfessional |
| NL-CM:11.1.1 | Vaccinatie | Vaccination | ProductCode+VaccinationDate | VaccinationDate | empty OR equal to Source | Patient | Administrator::Healthprofessional |
| NL-CM:12.4.1 | Bloeddruk | BloodPressure | BloodPressureDateTime | BloodPressureDateTime | empty OR equal to Source | Patient | Required |
| NL-CM:12.1.1 | Lichaamsgewicht | BodyWeight | WeightDateTime | WeightDateTime | empty OR equal to Source | Patient | Required |
| NL-CM:12.2.1 | Lichaamslengte | BodyHeight | HeightValue OR HeightDateTime | HeightDateTime | empty OR equal to Source | Patient | Required |
| NL-CM:14.1.1 | Verrichting | Procedure | Required | ProcedureStartDate | empty OR equal to Source | Patient | Performer::HealthProfessional |
| NL-CM:15.1.1 | Contact | Encounter | ContactType + StartDateTime | StartDateTime | empty OR equal to Source | Patient | ContactWith::HealthProfessional |
| NL-CM:17.1.1 | Zorgverlener | HealthProfessional | HealthProfessionalIdentificationNumber | Not Relevant | Not Relevant | HealthProfessional | Not Relevant |
Reconciliation
Metadata provides the opportunity for reconciliation: combining patient data from multiple sources in a consistent and integrated way. This may involve:
- receiving information, such as a BgZ, from another source and integrating that in one's own EHR;
- retrieving information from multiple sources, and showing that in a consistent and unambiguous way.
Reconciliation involves the following.
- De-duplication: data item X may be available from multiple sources, and should only be shown once. De-duplication is possible through consistent identifiers across data custodians.
- Semantic interpretation: data items from multiple sources should be classified in a consistent way, which is interoperable across sources. Semantic interoperability is possible through the use of consistent classification and terminologies. The HCIMs themselves, along with "Eenheid van Taal" (Unity of Language) terminology services already provide the necessary level of semantic interoperability.
- Provenance: it should be clear which data originates where, and the original source should be traceable and (where still possible) approachable. Provenance is achieved through authorship and information source.
- Attribution: patient data is a statement: usually a fact that something is the matter about something or someone at some point in time. Subject and date/time information allow the data item to be related to the real world.
Persistent identifiers
CDA has identifiers of the following form, where root must be an oid and extension is some local string value.:
<id extension="1A2B3C" root="2.16.840.1.113883.2.4.3.46.20"/>
FHIR has identifiers of the following form, where system is some uri and value is some local string value.:
<identifier>
<system value="http://www.example.org" />
<value value="1A2B3C" />
</identifier>
It is strongly recommended to use FHIR identifiers of the following form in the BgZ, where the oid is the same as the oid used in CDA-based exchanges. :
<identifier>
<system value="urn:oid:2.16.840.1.113883.2.4.3.46.20" />
<value value="1A2B3C" />
</identifier>
This greatly facilitates interoperability between FHIR and CDA.
|
When reconciling data from multiple sources, persistent identifiers MAY be used for the de-duplication of data. Systems may, however, choose to display duplicate data. |
|
When storing reconciled data from another source, persistent identifiers MUST be stored as well. |
Re-identification
When a record is received from another organization, it should be persisted with the identifiers of the source. However, when the record is updated, the Storing System:
- MUST issue a new Identifier, following their own identification mechanisms
- MUST assign its own professional and/or organization as Information Source
|
Example: a Patient is diagnosed at Hospital A by doctor X as having a Problem: Fracture of wrist, with a start date. The Patient is transferred to another hospital, and the BgZ is sent along. The Problem is there stored in the EHR with the original identifier, and doctor X from hospital A as Information Source. The Patient is treated, and the fracture is healed. Doctor Y changes the Problem to 'inactive', and adds an end date. The system in hospital B must now supply its own identifier, and record doctor Y from hospital B as Information Source. Doctor B, and hospital B are responsible for the entire new record. |
As of yet, HCIMs and the BgZ do not provide a reliable mechanism to record and exchange shared responsibility and treatment across organizations, and there is no way to record different individuals are responsible for different steps in the lifecycle of an HCIM. This should therefore not be attempted.
Provenance
Every data item is a statement, an assertion of a fact, recorded by someone somewhere. This original source should be retained when integrating data from multiple sources. The source can be:
- An individual as Information Source of the statement. This is the InformationSource from the zib. In CDA of FHIR this is the corresponding element when a person is populating this element. Examples:
- a health professional makes a diagnosis
- a patient reports alcohol and tobacco use
- An organization as Information Source of the statement. This is the InformationSource from the zib. In CDA of FHIR this is the corresponding element when an organization person is populating this element. Examples:
- a lab reports certain chemical or biological measurements on a specimen
- A XIS as the information source. This is the custodian a CDA document, and the XIS from which the HCIM was retrieved in FHIR (possibly other elements are suitable, as in XDS based exchanges or FHIR Documents). in Examples:
- a BgZ is received from a XIS, and the contact persons of the patient are listed without further author or source;
- a record is retrieved from a XIS, and the historical problems in the problem list do not have further author or source;
- lab results are retrieved from a XIS, but the lab where the measurements were made is not provided;
Certainly for historical data the original source of the statement often is not retained. The order above is the preferred order: i.e. when an individual is known as information source, provide that individual. In the case of an healthcare professional, provide the healthcare organization and professionals role as well. When no individual is known, use the organization. When no organization is known, use the originating XIS as source.
|
When displaying reconciled data from multiple sources, provenance metadata MUST be available to the person viewing that data. |
|
When storing reconciled data from another source, provenance metadata MUST be stored as well, in the order:
|
De-duplication
De-duplication is not always possible when receiving consecutive records with the same identifiers but different content. It may not always be obvious which of those records is the most recent one. This standard does not require specific mechanisms for de-deduplication, but suggests the following heuristics:
- when a record is received from A and B, and A is listed as the information source in both, assume the record from A to be the most recent one;
- when a record is received from A, stored, and retrieved again from A, assume the latter is most recent one;
- when a record is received from B and C, and A is listed as the information source in both, assume the most recently received record is most recent one.
A Processing System can flag such conflicting records. When in doubt, a medical professional should decide, possibly after consulting colleagues.
Implementation technologies
FHIR profiles
The FHIR profiles defined for MedMij are also used for the BgZ.
CDA templates
The entire publication can be found at Basisgegevensset Zorg 2017 (BgZ)
The following CDA templates are used for the BgZ 2017.
Infrastructure
This standard does not mandate a particular infrastructure, and therefore does not specify details for such an infrastructure.
Useful materials from other parties can be found on: